Recognizing and Managing Toxicities of Molecular Targeted Therapies for Colorectal Cancer
Traditional therapeutic concepts and treatment regimens for colorectal cancer are currently changing with the demonstration of the efficacy of biologic agents in this disease setting. The addition of the anti-vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab (Avastin) to conventional chemotherapy in the first- and second-line settings has shown a survival benefit; this outcome has helped to rapidly change the standard of care. Other targeted agents, such as anti-epidermal growth factor receptor (EGFR) antibodies, have shown proof of efficacy in colorectal cancer as well. The molecular targeted therapies are associated with toxicity profiles that are distinctly different from those seen with conventional chemotherapy. A notable difference is the absence of high risk for myelosuppression, diarrhea, or alopecia, which are common side effects of cytotoxic chemotherapy. This article will explore the toxicities associated with targeted therapies in detail in an attempt to provide assistance to the practicing oncologist in detecting and managing these side effects in their patients. In particular, the article will focus on the side effects associated with the three currently approved targeted drugs: the anti-VEGF monoclonal antibody bevacizumab and the anti-EGFR monoclonal antibodies cetuximab (Erbitux) and panitumumab (Vectibix).
ABSTRACT: Traditional therapeutic concepts and treatment regimens for colorectal cancer are currently changing with the demonstration of the efficacy of biologic agents in this disease setting. The addition of the anti-vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab (Avastin) to conventional chemotherapy in the first- and second-line settings has shown a survival benefit; this outcome has helped to rapidly change the standard of care. Other targeted agents, such as anti-epidermal growth factor receptor (EGFR) antibodies, have shown proof of efficacy in colorectal cancer as well. The molecular targeted therapies are associated with toxicity profiles that are distinctly different from those seen with conventional chemotherapy. A notable difference is the absence of high risk for myelosuppression, diarrhea, or alopecia, which are common side effects of cytotoxic chemotherapy. This article will explore the toxicities associated with targeted therapies in detail in an attempt to provide assistance to the practicing oncologist in detecting and managing these side effects in their patients. In particular, the article will focus on the side effects associated with the three currently approved targeted drugs: the anti-VEGF monoclonal antibody bevacizumab and the anti-EGFR monoclonal antibodies cetuximab (Erbitux) and panitumumab (Vectibix).
Until recently, systemic medical treatment of colorectal cancer was synonymous with the use of conventional chemotherapy. With the clear demonstration of the efficacy of biologic agents in this disease, traditional therapeutic concepts and treatment regimens are currently changing. In particular, the survival benefit observed with the addition of the anti-vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab (Avastin) to conventional chemotherapy in the first- and second-line settings has rapidly changed the standard of care.[1,2] In addition, other targeted agents such as anti-epidermal growth factor receptor (EGFR) antibodies have shown proof of efficacy in advanced colorectal cancer.[3,4] The practicing oncologist is now faced with the problem of how to best integrate these novel agents into treatment algorithms to maximize the therapeutic benefit for patients.
In addition, molecular targeted therapies exhibit toxicity profiles that are distinctly different from the adverse events associated with conventional chemotherapy. For instance, myelosuppressionone of the most common side effects of cytotoxic chemotherapyis not a characteristic of any of the targeted agents currently used in colorectal cancer. Likewise, these novel agents are not associated with a high risk of diarrhea or alopecia. They do, however, contain their own specific toxicity profiles with side effects that present a new learning experience to oncologists who have so far mainly dealt with conventional cytotoxic drugs.
This review tries to provide assistance in the detection and management of toxicities associated with molecular targeted agents in colorectal cancerin particular, the anti-VEGF monoclonal antibody bevacizumab and the anti-EGFR monoclonal antibodies cetuximab (Erbitux) and panitumumab (Vectibix). In addition, this article will comment and elaborate on the side effects listed in the package inserts of these three currently approved targeted drugs.
Anti-VEGF Therapy: Bevacizumab
See Table 1 for a list of the most pertinent toxicities associated with bevacizumab administration. In the following sections we will discuss several of these toxicities in detail.
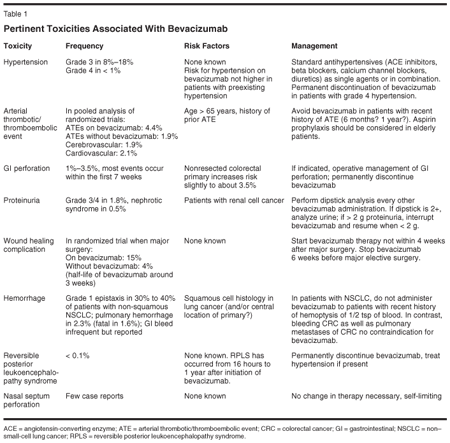
Hypertension
Hypertension is a class effect of all inhibitors of VEGF signaling. It is presumably due to decreased production and release of nitric oxide, a potent vasodilator, by endothelial cells.[5] Across clinical studies with bevacizumab, the incidence of National Cancer Institute Common Toxicity Criteria (NCI-CTC) grade 3 or 4 hypertension ranged from 8% to 18%.[6-10] Grade 3 is defined as hypertension requiring more than one antihypertensive drug or more intensive therapy than previously for blood pressure regulation; grade 4 is reserved for life-threatening consequences such as hypertensive crisis. The incidence of grade 4 hypertension in clinical trials with bevacizumab was less than 1%. While the onset of hypertension is normally gradual over several weeks, acute increases in blood pressure associated with initial or subsequent infusions of bevacizumab have been reported.
Medication classes used for management of patients with grade 3 hypertension receiving bevacizumab include standard antihypertensives such as angiotensin-converting enzyme (ACE) inhibitors, beta blockers, diuretics, and calcium channel blockers. In view of their documented renal-protective effects with decreased proteinuria, ACE inhibitors might be the preferred agents. Development or worsening of hypertension can require hospitalization or discontinuation of bevacizumab in up to 1.7% of patients. Hypertension can persist after discontinuation of bevacizumab, but will resolve in most patients after bevacizumab-containing therapy is terminated.
As with all grade 4 toxicities, bevacizumab should be permanently discontinued in patients with hypertensive crisis or hypertensive encephalopathy. It should be temporarily withheld in patients with severe hypertension that is not controlled with medical management.
Arterial Thrombotic/Thromboembolic Events
Arterial thrombotic/thromboembolic events (ATEs), which occurred at a higher incidence in patients receiving bevacizumab in combination with chemotherapy as compared to those receiving chemotherapy alone, included cerebral infarction, transient ischemic attacks, myocardial infarction, angina, and a variety of other events.
In a pooled analysis of randomized, controlled clinical trials in three different indications (breast, lung, and colorectal cancer) involving 1,745 patients, the incidence of ATEs was 4.4% among patients treated with bevacizumab in combination with chemotherapy and 1.9% among patients receiving chemotherapy alone. Fatal outcomes for these events occurred in 7 of 963 patients (0.7%) who were treated with bevacizumab in combination with chemotherapy, compared to 3 of 782 patients (0.4%) who were treated with chemotherapy alone. The incidence of both cerebrovascular arterial events (1.9% vs 0.5%) and cardiovascular arterial events (2.1% vs 1.0%) was increased in patients receiving bevacizumab compared to chemotherapy alone.
The relative risk of ATEs was greater in patients 65 and over (8.5% vs 2.9%) as compared to those younger than 65 (2.1% vs 1.4%). For patients over 65 who also had a history of prior ATE, the risk for another ATE on bevacizumab was 17.9% compared with 2.2% for chemotherapy alone. It has to be noted, though, that this ATE high-risk group of patients showed the same clinical benefit from treatment with bevacizumab in terms of progression-free and overall survival as all unselected patients in the pivotal trial by Hurwitz et al. Thus, routinely withholding bevacizumab in high-risk patients would deny them a potentially relevant clinical benefit. Therefore, the use of bevacizumab in patients with higher risk for ATEs should be individually discussed rather than routinely withheld. Since the safety of bevacizumab in combination with anticoagulants such as aspirin and even warfarin sodium has been well established, the prophylactic use of aspirin in patients with a risk profile for arterial events on bevacizumab should be considered.
The safety of resumption of bevacizumab therapy after resolution of an ATE has not been studied. Bevacizumab should be permanently discontinued in patients who experience a severe ATE on treatment.
Gastrointestinal Perforation
Gastrointestinal perforation (GIP) is a side effect that was first reported in the pivotal trial in colorectal cancer leading to the approval of bevacizumab as a component of first-line therapy. Of 402 patients, 6 (1.5%) on IFL (irinotecan [Camptosar], fluorouracil [5-FU], leucovorin) plus bevacizumab experienced a GIP; some of these patients resumed treatment with bevacizumab in this placebo-controlled trial in ignorance of the causality of the GIPs. Since then GIP has been clearly associated with the use of bevacizumab, not only in patients with colorectal cancer, but also in those with other gastrointestinal and nongastrointestinal malignancies.
In advanced colorectal cancer, the incidence of GIP has been reported between 1.2% and 4.2%,[8,11] most commonly in the range between 1.5% and 2%. In order to capture more patients who experienced this relatively rare but serious complication, two observational studies were initiated: the BRiTE trial[12] in the United States and the First-BEAT study[11] in Europe. Both studies, which combined included more than 3,700 patients on bevacizumab, confirmed the incidence of GIP as around 1.5%. No specific risk factors (concomitant medication, prior endoscopy, etc) for a GIP on therapy were identified, except for the fact that the presence of an unresected primary tumor was associated with a slightly higher risk of GIP (in BRiTE, 3.3% vs 1.4%).
The typical presentation of GIP was reported as abdominal pain associated with symptoms such as constipation and emesis. These episodes occurred with or without intra-abdominal abscesses and at various time points during treatment ranging from 1 week to greater than 1 year from initiation of bevacizumab, with most events occurring within the first 50 days.
Proteinuria
In the initial randomized phase II study in patients with advanced colorectal cancer, urine dipstick analysis revealed some proteinuria in about 25% of patients on bevacizumab. In the pivotal first-line trial, the incidence of NCI-CTC grade 3 proteinuria, characterized as > 3.5 g/24 hours, was 0.8% in bevacizumab-treated patients; no patient experienced grade 4 proteinuria (nephrotic syndrome). The bevacizumab package insert cites that nephrotic syndrome occurred in 7 of 1,459 (0.5%) patients receiving bevacizumab in clinical studies. One of these patients died and one required dialysis. In three patients, proteinuria decreased in severity several months after discontinuation of bevacizumab. No patient had normalization of urinary protein levels (by 24-hour urine) following discontinuation of bevacizumab. The highest incidence of proteinuria was observed in patients with metastatic renal cell carcinoma, an indication for which bevacizumab is currently not approved.
In routine practice, patients on bevacizumab should be monitored by monthly urine dipstick analyses. A 24-hour urine should be analyzed for proteinuria in patients with 2+ dipstick results. In most clinical studies, bevacizumab was interrupted for > 2 g of proteinuria/24 hours and resumed when proteinuria was < 2 g/24 hours. In patients with nephrotic syndrome, bevacizumab should be discontinued. The pathomechanism of proteinuria on bevacizumab is still unclear, but analogies with the finding of circulating antiangiogenic factors in pre-eclampsia are intriguing.[13]
Delayed Wound Healing
Since bevacizumab was found to impair wound healing in animal models, in all clinical studies of bevacizumab patients were not allowed to receive bevacizumab within the first 28 days after major surgery. In the Hurwitz trial, 29 patients on IFL alone and 75 patients on IFL plus bevacizumab underwent invasive surgery. The risk of grade 3 or 4 wound healing complications was 3.4% and 13%, respectively. The relatively long half-life of bevacizumab around 3 weeks has led to the recommendation to defer elective major surgical interventions for at least 6 weeks after the last dose of bevacizumab, thus respecting about two half-lives of the drug. This recommendation is of particular importance for patients scheduled to undergo potentially curative metastasectomy in the form of liver resection after neoadjuvant, bevacizumab-containing chemotherapy. In clinical practice, neoadjuvant treatment such as FOLFOX (5-FU, leucovorin, oxaliplatin [Eloxatin]) plus bevacizumab should omit bevacizumab for the last one to two cycles before planned liver surgery. It should be emphasized that minor surgical interventions such as port-a-cath implantations can safely be performed on bevacizumab; of course, bevacizumab treatment presents only a relative and no absolute contraindication for surgery, in particular, in case of surgical emergencies such as bowel perforations.
Hemorrhage
Two distinct patterns of bleeding have occurred in patients receiving bevacizumab. The first is minor hemorrhage, most commonly NCI-CTC grade 1 epistaxis, which affects about one-third of all patients. The second is rare, but serious (and in some cases fatal) hemorrhagic events, which mainly affect patients with non-small-cell lung cancer (NSCLC)predominantly those with squamous cell histology.
In a randomized phase II trial in patients with NSCLC, 4 of 13 (31%) bevacizumab-treated patients with squamous cell histology and 2 of 53 (4%) bevacizumab-treated patients with histology other than squamous cell experienced serious or fatal pulmonary hemorrhage as compared to none of the 32 (0%) patients receiving chemotherapy alone.[14] Of the patients experiencing pulmonary hemorrhage requiring medical intervention, many had cavitation and/or necrosis of the tumor, either preexisting or developing during bevacizumab therapy.
In the pivotal phase III trial that excluded patients with tumors of squamous cell histology and eventually led to the approval of bevacizumab in NSCLC, the rate of pulmonary hemorrhage requiring medical intervention for the paclitaxel/carboplatin (PC) plus bevacizumab arm was 2.3% (10 of 427) compared to 0.5% (2 of 441) for the PC alone arm.[15] There were seven deaths due to pulmonary hemorrhage reported by investigators in the PC plus bevacizumab arm as compared to one in the PC alone arm. Generally, these serious hemorrhagic events presented as major or massive hemoptysis without an antecedent history of minor hemoptysis during bevacizumab therapy.
Based on these experiences, bevacizumab should not be administered to patients with a recent history of hemoptysis of more than half a teaspoon of red blood. It is of note that no increased risk of pulmonary hemorrhage on bevacizumab has been observed in patients with pulmonary metastasis of nonlung primaries such as colorectal cancer.
Other serious bleeding events occurring in patients receiving bevacizumab across all indications include gastrointestinal hemorrhage, subarachnoid hemorrhage, and hemorrhagic stroke. Some of these events were fatal.
The risk of central nervous system (CNS) bleeding in patients with CNS metastases receiving bevacizumab has not been evaluated because these patients were excluded from late-stage clinical studies following development of CNS hemorrhage in a patient with a CNS metastasis in a phase I study.
Reversible Posterior Leukoencephalopathy Syndrome
Reversible posterior leukoencephalopathy syndrome (RPLS), which has been reported in clinical studies (with an incidence of < 0.1%) and in post-FDA (US Food and Drug Administration) approval experience, has recently been included in the Avastin package insert.[16] It is a neurologic disorder that can present with headache, seizure, lethargy, confusion, blindness, and other visual and neurologic disturbances in association with posterior cerebral white matter edema. Mild to severe hypertension may be present, but is not necessary for diagnosis of RPLS. Magnetic resonance imaging is necessary to confirm diagnosis. Reversible posterior leukoencephalopathy syndrome has been associated with a great variety of pathologic conditions such as hypertension, preeclampsia, connective tissue diseases, and conventional chemotherapy for hematologic and nonhematologic malignancies. In patients treated with bevacizumab, the onset of symptoms has been reported to occur from 16 hours to 1 year after initiation of bevacizumab. With appropriate management, RPLS is reversible in the majority of cases.
In patients developing RPLS, bevacizumab should be discontinued and treatment of hypertension, if present, needs to be initiated. Symptoms usually resolve or improve within days, although some patients have persistent neurologic sequelae. To date, the safety of reinitiating bevacizumab therapy in patients previously experiencing RPLS is not known.
Nasal Septum Perforation
Most recently, case reports of nasal septum perforations in patients on bevacizumab have emerged.[17] While the exact mechanism for this rare phenomenon is unclear it is conceivable that chemotherapy-induced mucosal irritations in form of micro-breaks and ulcerations in combination with the well-known inhibition of wound healing by bevacizumab could be responsible for the development of nasal septum perforations. The consensus so far is that these perforations are self-limiting and do not require any treatment modifications. Patients should be advised to avoid intranasal manipulations on bevacizumab therapy.
Anti-EGFR Therapy: Cetuximab and Panitumumab
Cutaneous Reactions
See Table 2 for a list of cutaneous reactions associated with EGFR inhibitors.
The skin toxicity associated with all EGF-targeting agents, monoclonal antibodies against the EGF receptor, or EGF receptor tyrosine kinase inhibitors is closely linked to their mechanism of action. It is self-evident that the skin contains an abundance of epidermal growth factor receptors and is thus affected by any EGFR-targeting agent. Histologic data from skin biopsy specimens indicate that rash is a class effect of EGFR-targeted agents.[18] A neutrophilic infiltrate has been reported in the dermal tissue, particularly the infundibular part of the hair follicle, but the sebaceous glands are not affected. This finding clearly distinguishes the EGFR rash from conventional acne. In addition, the stratum corneum layer of the epidermis is thinner, more compact, and has lost its basket-weave pattern in skin.[18] In various clinical settings, the occurrence and severity of the EGFR rash has been correlated with a better outcome on therapy with EGFR inhibitors.[3,4,19]

Skin reactions generally occur within the first 2 weeks of therapy with anti-EGFR agents and reach maximum intensity after 2 to 3 weeks. Subsequently, the rash component of the skin toxicity shows spontaneous improvement, with generalized skin dryness (xerosis) frequently becoming the dominant symptom.
As mentioned before, the pathomechanism of the EGFR rash differs from normal acne and therefore, therapeutic approaches used for acne do not necessarily have a rationale in the management of the EGFR rash. In order to avoid confusion, the EGFR rash should not even be termed "acne-like," but rather be referred to as a follicular eruption that can resemble pustular-appearing lesions. Locations most commonly include the face, scalp, and upper torso, and can sometimes extend to the extremities.
By far the majority of patients on EGFR-targeted agents will experience some form of skin toxicity. In clinical studies, grade 1 to 4 skin toxicity was reported in 70% to 100% of patients.[20] In patients with advanced colorectal cancer, EGFR rash of any grade was seen in 88% of patients on cetuximab and 90% of patients on panitumumab with severe (grade 3 or 4) rash reported in 12% and 16% of patients, respectively.[3,4]
NCI-CTC scales (Table 3) provide various ways to classify a skin rash, adding some complexity to the way this characteristic toxicity is recorded-apart from the obvious subjective bias in classifying the severity of a rash in general. This has to be kept in mind when the results of clinical trials and even the information in package inserts of cetuximab and panitumumab are compared.
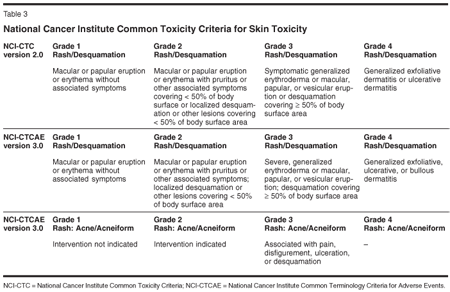
Although the EGFR rash is by far the most common side effect of anti-EGFR therapy, to date no consensus has been achieved on how to manage patients who experience severe skin toxicity; any therapeutic approach is quite empirical. This frustrating lack of evidence is filled by a plethora of anecdotal reports of success with various treatments, including retinoids, oral and topical steroids (in direct contradiction to the Erbitux package insert), emollients, and antibiotics. Rash superinfection might respond to topical clindamycin or short courses of oral tetracyclines, amoxicillin/clavulanic acid, or cephalosporins.[18,20] Even topical immunomudulators have been successfully applied in form of tacrolimus (Protopic) ointment and pimecrolimus (Elidel) cream.[21]
However, there are no controlled data to show that any topical applications or oral medications are useful for managing rash, and rash often improves spontaneously without medical intervention. This has to be taken into account when successful therapeutic interventions based on individual experience or based on small patient cohorts are reported. Patients with severe skin toxicities on EGFR inhibitors should be referred to a dermatologist in an interdisciplinary approach.
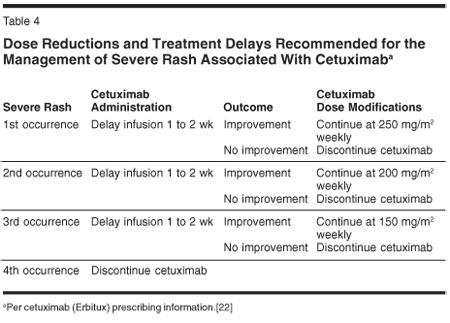
In addition, the current prescribing information for Erbitux recommends specific dose reductions and treatment delays as part of the management of severe rash in patients on cetuximab (Table 4).[22] The package insert for Vectibix calls for withholding the drug for grade 3 and higher dermatologic toxicities or for toxicities that are considered intolerable.[23] If the toxicity does not improve to grade 2 or better, panitumumab should be discontinued permanently. Otherwise, treatment should be resumed at 50% of the original dose of panitumumab, andif toleratedsubsequent doses of the drug can be increased in 25% increments until the original recommended dose of 6 mg/kg every 2 weeks is reached.
Periungual and ungual toxicities are common phenomena in patients on EGFR inhibitors in the form of periungual cracks, pustules, and honey crusting, and are mainly found in the context of a follicular eruption. Patients on EGFR targeted therapy should be instructed to pay attention to nail care and to avoid trauma such as nail biting, pushing back of cuticles, and tight shoes.
Apart from skin toxicities, ocular toxicities such as conjunctivitis and blepharitis with increased lacrimation have been reported for both monoclonal antibodies.[4,24] In addition, stomatitis and oral mucositis can occur in about 5% of patients.
Various hair changes have been reported in the form of fine, brittle, or curly hair and slowed hair growth, as well as hypertrichosis and trichomegaly (elongated eyelashes),[25] which might require trimming to avoid corneal abrasions.
Hypersensitivity
(Infusion Reactions)
One of the key side effects noted in early trials with cetuximab was the occurrence of hypersensitivity infusion reactions in about 20% of patients (all grade) with about 3% of patients experiencing grade 3 or 4 reactions, most commonly (in about 90%) associated with the very first dose of the drug. Consequently, the package insert recommends an obligatory premedication with an H2 antagonist such as diphenhydramine before cetuximab administrations.[22] Most recently, these recommendations have been questioned since in a large-volume institution premedication was omitted after two uncomplicated doses of cetuximab without provoking infusion reactions.[26]
Since cetuximab as a chimeric mouse-human antibody contains a significant mouse component in its immunoglobulin backbone, the hypersensitivity reactions observed were thought to be mediated by preformed human-antimouse antibodies. While so far no direct evidence for this assumption has been revealed, the findings of a significantly lower rate of severe infusion reaction with the fully human monoclonal antibody panitumumab can be used as indirect evidence supporting this hypothesis.
In the pivotal trial leading to the FDA approval of panitumumab as single-agent salvage therapy in colorectal cancer, no grade 3 or 4 infusion reaction was reportedwithout mandatory premedication.[4] The expanded definition of infusion reactions used to generate the text of the official Vectibix prescribing information quotes 1% as the rate of severe infusions reactions (precisely 6 of 1,336 patients, 0.4%), with one patient permanently discontinuing panitumumab due to a serious reaction.[23] These data clearly demonstrate that the rate of severe hypersensitivity reactions on panitumumab is extremely low so that no mandatory premedication with antihistaminic agents is required.
Pulmonary Toxicity
Pulmonary toxicity in the form of interstitial lung disease (ILD) has been reported with cetuximab and panitumumab, as well as with gefitinib (Iressa) and erlotinib (Tarceva) as EGFR tyrosine kinase inhibitors. Thus, ILD should be regarded as a class effect of EGFR-targeted agents. Interstitial lung disease appears in the form of an interstitial fibrosis with clinical symptoms of wheezing, dry cough, and hypoxia. The onset of symptoms varies between 1 week and several months after initiation of therapy. The clinical course can lead to rapid lung failure with fatalities reported in clinical trials and post-FDA approval. Patients with a history of interstitial pneumonia, pulmonary fibrosis, and interstitial pneumonitis might be at higher risk for severe ILD. Any new pulmonary symptom on EGFR targeted therapy should be followed by chest CT scan to exclude the presence of ILD. In patients who develop ILD, EGFR inhibitors should be permanently discontinued.
Hypomagnesemia
Recent clinical observations led to the discovery of a clinically relevant side effect of anti-EGFR monoclonal antibodies: hypomagnesemia. This phenomenon was first reported in a young patient with advanced CRC who developed profound fatigue and symptomatic hypocalcemia and hypomagnesemia while on cetuximab plus irinotecan therapy.[27] A subsequent retrospective review of laboratory values confirmed lower serum magnesium levels in about 22% of patients of cetuximab.[27] In the phase III panitumumab trial, 39% of patients developed lower magnesium levels of any grade with 4% experiencing grade 3 or 4 hypomagnesemia that required oral or IV substitution of magnesium in 2%.
EGFR is strongly expressed in the kidney, particularly in the ascending limb of the loop of Henle where 70% of filtered magnesium is reabsorbed. EGFR blockade by monoclonal antibodies may interfere with magnesium transport and thus lead to magnesium depletion. Serum magnesium levels should be routinely measured on therapy with cetuximab and panitumumab and hypomagnesemia should be considered in patients who develop fatigue and muscle weakness on therapy. If indicated magnesium supplementation can readily reverse clinical symptoms, but might have to be continued beyond the discontinuation of the EGFR antibody therapy.
Conclusion
Novel targeted approaches, in particular, anti-VEGF and anti-EGFR agents, have in many ways lived up to their promise of improving the efficacy of our treatment options in colorectal cancer. While the word "targeted" might have promised a more selective effect on tumor vs normal tissue, these agents have, in fact, come with the price of new side effects that are in some instances intrinsically linked to their mechanism of action and thus almost impossible to avoid. Some of the side effects will in clinical practice be a limiting factor for the use of these novel agents. A better understanding of the mechanism of action of any targeted agent, in particular, their effects on normal, nonmalignant tissue, will hopefully provide us with more rationale approaches to ameliorate or prevent agent-specific toxicities in the future.
Disclosures:
Dr. Grothey has acted as a consultant for and received honoraria from Genentech, Bristol-Myers Squibb, and Amgen.
References:
1. Hurwitz H, Fehrenbacher L, Hainsworth J, et al: Bevacizumab in combination with 5-fluorouracil and leucovorin: A promising regimen for first-line metastatic colorectal cancer (abstract 286). 2004 ASCO Gastrointestinal Cancers Symposium. San Francisco, 2004.
2. Giantonio B, Catalano D, Meropol NJ, et al: High-dose bevacizumab improves survival when combined with FOLFOX4 in previously treated advanced colorectal cancer: Results from the Eastern Cooperative Oncology Group (ECOG) study E3200. J Clin Oncol 23:2, 2005.
3. Cunningham D, Humblet Y, Siena S, et al: Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337-345, 2004.
4. Peeters M, Van Cutsem E, Siena S, et al: A phase 3, multicenter, randomized controlled trial (RCT) of panitumumab plus best supportive care (BSC) vs BSC alone in patients (pts) with metastatic colorectal cancer (mCRC) (abstract CP-1). American Association for Cancer Research. Washington, DC, 2006.
5. Wang Y, Fei D, Vanderlaan M, et al: Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis 7:335-345, 2004
6. Fuchs C, Marshall J, Mitchell E, et al: A randomized trial of first-line irinotecan/fluoropyrimidine combinations with or without celecoxib in metastatic colorectal cancer (BICC-C) (abstract 3506). J Clin Oncol 24(suppl 18S):147s, 2006.
7. Giantonio BJ, Catalano PJ, Meropol NJ, et al: High-dose bevacizumab improves survival when combined with FOLFOX4 in previously treated advanced colorectal cancer: Results from the Eastern Cooperative Oncology Group (ECOG) study E3200 (abstract 2). J Clin Oncol 23(suppl 16S):1s, 2005.
8. Hochster HS, Hart LL, Ramanathan RK, et al: Safety and efficacy of oxaliplatin/fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer (mCRC): Final analysis of the TREE-Study (abstract 3510). J Clin Oncol 24(suppl 18S):148s, 2006.
9. Hurwitz H, Fehrenbacher L, Novotny W, et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335-2342, 2004.
10. Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al: Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21:60-65, 2003.
11. Berry SR, Cunningham D, Michael M, et al, on behalf of the First BEAT investigators: Preliminary safety of bevacizumab with first-line Folfox, Capox, Folfiri, and capecitabine for mCRC-First B.E.A. Trial (abstract 3534). J Clin Oncol 24(suppl 18S):154s, 2006.
12. Sugrue M, Kozloff M, Hainsworth J, et al: Risk factors for gastrointestinal perforations in patients with metastatic colorectal cancer receiving bevacizumab plus chemotherapy (abstract 3535). J Clin Oncol 24(suppl 18S):154s, 2006.
13. Karumanchi SA, Maynard SE, Stillman IE, et al: Preeclampsia: A renal perspective. Kidney Int 67:2101-2113, 2005.
14. Johnson DH, Fehrenbacher L, Novotny WF, et al: Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22:2184-2191, 2004.
15. Sandler AB, Gray R, Brahmer J, et al: Randomized phase II/III trial of paclitaxel (P) plus carboplatin (C) with or without bevacizumab (NSC #704865) in patients with advanced non-squamous non-small cell lung cancer (NSCLC): An Eastern Cooperative Oncology Group (ECOG) Trial E4599 (abstract LBA4). J Clin Oncol 23(suppl 16S):2s, 2005.
16. Genentech: Avastin prescribing information, 2006. http://www.gene.com/gene/products/information/oncology/avastin/insert.jsp
17. Fakih MG, Lombardo JC: Bevacizumab-induced nasal septum perforation. Oncologist 11:85-86, 2006.
18. Perez-Soler R, Saltz L: Cutaneous adverse effects with HER1/EGFR-targeted agents: Is there a silver lining? J Clin Oncol 23:5235-5246, 2005.
19. Xiong HQ, Rosenberg A, LoBuglio A, et al: Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: A multicenter phase II trial. J Clin Oncol 22:2610-2616, 2004.
20. Fox LP: Pathology and management of dermatologic toxicities associated with anti-EGFR therapy. Oncology (Williston Park) 20(suppl 2):26-34, 2006.
21. Lacouture ME, Basti S, Patel J, et al: The SERIES clinic: An interdisciplinary approach to the management of toxicities of EGFR inhibitors. J Support Oncol 4:236-238, 2006.
22. Bristol-Myers Squibb: Erbitux prescribing information, 2006. http://www.erbitux.com
23. Amgen: Vectibix prescribing information, 2006. http://www.amgen.com/medpro/vectibix_pi.html
24. Dranko S, Kinney C, Ramanathan RK: Ocular toxicity related to cetuximab monotherapy in patients with colorectal cancer. Clin Colorectal Cancer 6:224-225, 2006.
25. Montagut C, Grau JJ, Grimalt R, et al: Abnormal hair growth in a patient with head and neck cancer treated with the anti-epidermal growth factor receptor monoclonal antibody cetuximab. J Clin Oncol 23:5273-5275, 2005.
26. Timoney J, Chung KY, Park V, et al: Cetuximab use without chronic antihistamine premedication (abstract 13521). J Clin Oncol 24(suppl 18S), 2006.
27. Schrag D, Chung KY, Flombaum C, et al: Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst 97:1221-1224, 2005.