Treatment of Metastatic Colorectal Cancer: From Cytotoxic Agents to Molecular Agents and Multitargeted Strategies
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related death in United States. For nearly 50 years, fluorouracil has been the only anticancer drug proven to benefit patients with metastatic CRC (mCRC), and it continues to be the backbone on which most treatment regimens are built. In the past 10 years, development of the topoisomerase I inhibitor irinotecan (Camptosar), the third-generation platinum analog oxaliplatin (Eloxatin), and the oral fluoropyrimidine capecitabine (Xeloda) advanced mCRC treatment and opened up an era of combination chemotherapy. More recently, monoclonal antibodies such as bevacizumab (Avastin), cetuximab (Erbitux), and panitumumab (Vectibix) have become available for use in mCRC treatment in combination with cytotoxic agents and as monotherapies. The addition of these targeted agents to the mCRC treatment armamentarium has resulted in more therapeutic options and improved treatment outcomes for the patients. The prospect of mCRC treatment is ever promising as more targeted agents such as vatalanib are being introduced and as intelligent combination regimens are being designed based upon a better understanding of pharmacokinetics. In this article we review various treatment options, including cytotoxic and targeted agents, currently available for patients with mCRC.
ABSTRACT: Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related death in United States. For nearly 50 years, fluorouracil has been the only anticancer drug proven to benefit patients with metastatic CRC (mCRC), and it continues to be the backbone on which most treatment regimens are built. In the past 10 years, development of the topoisomerase I inhibitor irinotecan (Camptosar), the third-generation platinum analog oxaliplatin (Eloxatin), and the oral fluoropyrimidine capecitabine (Xeloda) advanced mCRC treatment and opened up an era of combination chemotherapy. More recently, monoclonal antibodies such as bevacizumab (Avastin), cetuximab (Erbitux), and panitumumab (Vectibix) have become available for use in mCRC treatment in combination with cytotoxic agents and as monotherapies. The addition of these targeted agents to the mCRC treatment armamentarium has resulted in more therapeutic options and improved treatment outcomes for the patients. The prospect of mCRC treatment is ever promising as more targeted agents such as vatalanib are being introduced and as intelligent combination regimens are being designed based upon a better understanding of pharmacokinetics. In this article we review various treatment options, including cytotoxic and targeted agents, currently available for patients with mCRC.
Colorectal cancer (CRC) is the third most common cancer, with an estimated 150,000 new cases diagnosed each year. More than 55,000 CRC-related deaths are expected in 2006, making it the second leading cause of cancer-related death in United States.[1] While surgical resection is the curative modality for localized CRC, up to 40% of CRC patients have distant metastasis at the time of diagnosis.[1] Except for the patients who are candidates for resection of liver metastasis, the approach in treating metastatic colorectal cancer (mCRC) patients is palliative systemic chemotherapy.
For nearly 50 years, fluorouracil (5-FU) has been the only active anticancer agent in CRC providing a modest clinical benefit (median overall survival [OS] of 6 to 8 months).[2] In the past 10 years, development of the topoisomerase I inhibitor irinotecan (Camptosar), the third-generation platinum analog oxaliplatin (Eloxatin), and the oral fluoropyrimidine capecitabine (Xeloda) changed the landscape of mCRC treatment. Optimizing the combination of these agents has improved the outlook of patients with mCRC, resulting in median overall survival consistently reaching approximately 20 months.
More recently, the development of targeted agents such as bevacizumab (Avastin), cetuximab (Erbitux), and panitumumab (Vectibix) advanced the treatment of mCRC even further. These monoclonal antibodies gave rise to more therapeutic options for oncologists and improved outcomes for mCRC patients, as evidenced by an impressive median OS of 25.1 months reported in a study that added bevacizumab to IFL (irinotecan/5-FU/leucovorin)[3] (Figure 1). The future outlook on treatment of mCRC is ever promising as more targeted agents (vatalanib, etc) are being introduced and more sophisticated combination regimens are being developed to optimize anticancer therapy. In this article, we review the use of cytotoxic chemotherapeutic agents and targeted agents in patients with mCRC.
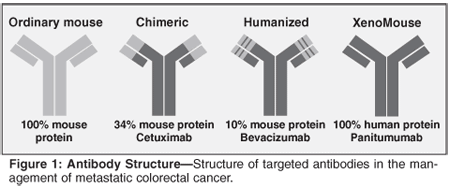
Cytotoxic Agents
Fluorouracil
Fluorouracil, a fluoropyrimidine analog that inhibits thymidylate synthase (TS) and ultimately inhibits DNA synthesis and function,[3a] had been the only anticancer drug proven to benefit patients with mCRC for 50 years. It has also been the backbone on which most regimens for treatment of mCRC are built.[5] When used alone, 5-FU has limited activity in advanced disease, rendering a response rate (RR) ranging from 10% to 15%. A meta-analysis of data incorporating 3,300 patients from 19 clinical trials demonstrated that combining leucovorin (LV), a biomodulating agent, with 5-FU was associated with improved response rates up to 21% from 11% (P < .0001) and improved survival from 10.5 to 11.7 months (P < .004).[2,5,6] The 5-FU/LV regimen was recognized as standard first-line treatment of mCRC until more advanced combination therapies containing irinotecan and oxaliplatin were developed.[7] However, optimizing ways in which 5-FU/LV is delivered remains quite debatable.
Among several different regimens, the most common bolus schedules are as follows: The Mayo Clinic regimen with 5-FU at a dose of 425 mg/m2 and LV at 20 mg/m2 every 4 weeks, and a weekly schedule developed by Roswell Park Cancer Institute with 5-FU at 600 mg/m2 and LV at 500 mg/m2 weekly × 6 out of 8 weeks. Two randomized trials have demonstrated similar response rates, duration of response, pregression-free intervals, and median overall survival between the monthly and weekly schedules of LV-modulated 5-FU.[8,9]
The clinical efficacy of 5-FU/LV has been improved upon by the use of infusional schedules. A meta-analysis that incorporated 1,219 patients from six different randomized trials compared infusional 5-FU with bolus 5-FU, and revealed that infusional schedules resulted in higher response rates than bolus schedules of 5-FU (22% vs 14%; P < .0002). Infusional schedules were also associated with less hematologic toxicity compared to bolus regimens (4% vs 31%, respectively; P < .0001) and less gastrointestinal toxicity.[10]
Common infusional schedules include the protracted venous infusion (PVI) schedule with 5-FU at 300 mg/m2 for 28 days; the AIO German regimen of 5-FU at 2,000 to 2,600 mg/m2 over 24 hours with LV at 500 mg/m2 administered once weekly for 6 weeks with 1 week rest; LV5FU2 (the de Gramont regimen), which is a combination of bolus and infusional 5-FU/LV-administered on days 1 and 2 on a biweekly schedule; and a simplified LV5FU2 schedule with LV at
400 mg/m2 and 5-FU at 400 mg/m2 on day 1, followed by a single 46-hour continuous infusion of 5-FU for a total infusion dose of 2,400 mg/m2.[10-13] Based on recent National Comprehensive Cancer Network (NCCN) guidelines, PVI 5-FU with or without LV is a reasonable first-line treatment option for patients who cannot tolerate intensive chemotherapy.[7]
Capecitabine
Capecitabine is an oral fluoropyrimidine carbamate prodrug of 5-FU designed to be reliably absorbed intact through the gastrointestinal tract. It then becomes activated by three successive enzymatic steps. The enzyme thymidylate phosphorylase (TP) is involved in the final step, and it is expressed at a higher level in tumor tissue compared to corresponding normal tissue. This tumor localization of TP may be the basis for the selective activation of capecitabine in tumors and associated favorable tolerability.[14,15]
In two randomized phase III trials comparing capecitabine with the Mayo Clinic regimen of bolus 5-FU/LV, capecitabine showed similar efficacy.[16,17] An integrated analysis of the two trials revealed that the overall response rate was higher with capecitabine than with bolus 5-FU/LV (25.7% vs 16.7%; P < .0002), while time to progression (TTP) and survival were similar. Capecitabine was also associated with fewer side effects. Gastrointestinal and hematologic toxicities were significantly lower in incidence for the capecitabine arm compared to the bolus 5-FU/LV arm. Hand-foot syndrome was the only side effect that was observed with higher incidence on the capecitabine arm. There has not been any randomized clinical trial that compared the efficacy and toxicity of capecitabine with any infusional 5-FU schedules. When compared to historical controls of continuous infusion of 5-FU, capecitabine monotherapy appeared to have similar response rate, time to progression, and median survival.[18]
Capecitabine, at a dose of 1,250 mg/m2 twice daily on a 14 out of every 21 days cycle, was approved in the United States for first-line treatment of mCRC when fluoropyrimidine monotherapy is indicated. However, clinical experience and retrospective analysis indicate that 900 to 1,000 mg/m2 twice daily may be better tolerated with similar efficacy.[18]
Irinotecan
Irinotecan is a topoisomerase I inhibitor.[19] Irinotecan monotherapy in 5-FU-refractory mCRC patients showed an improved 1-year overall survival (36 % vs 14 %) and improved quality of life when compared with best supportive care. Severe diarrhea is the well-known dose-limiting toxicity of irinotecan.[20] Irinotecan was initially approved for second-line treatment by the US Food and Drug Administration (FDA); however, three randomized phase III trials provided evidence that the first-line combination regimen including irinotecan and 5-FU/LV rendered improved clinical efficacy, including overall survival, compared to 5-FU/LV alone.[21-23]
In Europe, Douillard et al compared the clinical efficacy of infusional 5-FU plus irinotecan vs infusional 5-FU/LV alone. In this trial, the irinotecan group was superior in RR (35% vs 22%; P = .005), time to treatment failure (TTF, 6.7 vs 4.4 months; P = .001), and OS (17.4 vs 14.2 months; P = .031).[21] Kohne et al compared weekly infusional 5-FU/LV with and without irinotecan and reported that the addition of irinotecan improved RR (54% vs 31.5%; P < .0001) and time to progression (TTP, 8.5 vs 6.4 months; P = .0001). However, the median survival benefit (20.1 vs 16.9 months; P = .2279 log-rank) did not reach the level of statistical significance[22] (Table 1).
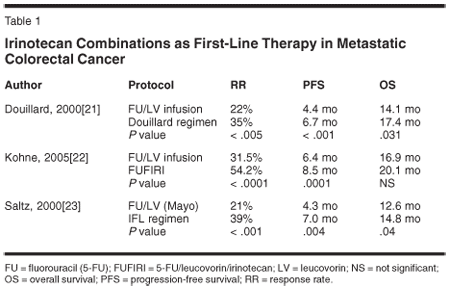
In the United States, Saltz et al compared IFL (bolus 5-FU/LV) on a weekly schedule vs 5-FU/LV alone, and reported the superiority of the IFL regimen over 5-FU/LV alone in terms of RR (39% vs 21%; P < .001), median progression-free survival (PFS, 7.0 vs 4.3 months; P = .004), and OS (14.8 vs 12.6 months; P = .04).[23]
The weekly IFL regimen resulted in an increased incidence of diarrhea, dehydration, and myelosuppression when compared to the infusional schedules of 5-FU/LV (FOLFIRI or the Douillard regimen).[21] Based on this observation the IFL regimen, which was preferred among US oncologists due to their familiarity with it, is now used rarely or with modification consisting of 2 weeks on/1 week off. In contrast, FOLFIRI strategies have recently been embraced by more US oncologists. In fact, based on a recent randomized trial by Fuchs et al, even the modified IFL regimen (m-IFL) should be considered obsolete, and the FOLFIRI regimen should be the regimen of choice when 5-FU is combined with irinotecan in treatment of previously untreated mCRC. In this trial, FOLFIRI showed its superiority over m-IFL with respect to TTP (8.2 vs 6.0 months; P = .01) and median OS (23.1 vs 17.6 months; P = .10). FOLFIRI was also better tolerated than m-IFL [24]
Irinotecan + Capecitabine (CapeIri)
Investigators are actively evaluating the combination of irinotecan/capecitabine to take advantage of the convenience and tolerability of the oral capecitabine. Numerous phase II studies have shown the efficacy of CapeIri with a manageable side-effect profile.[25-27] In one phase II study including 47 patients with metastatic or unresectable colorectal cancer, Ahn et al investigated the combination of capecitabine (1,000 mg/m2 orally twice daily on days 2-15 of a 3-week cycle) plus irinotecan (100 mg/m2 IV on days 1 and 8). This trial reported an overall RR of 51.4% (19 partial responses [PRs]; 95% confidence interval [CI] = 35.3%-67.5%), a median TTP of 7.1 months (95% CI = 4.6-9.6 months), and a median OS of 24.8 months (95% CI = 10.5-39.1 months). This regimen was relatively well-tolerated, with 24% grade 3/4 diarrhea and 11% grade 3/4 neutropenia. There were no treatment-related deaths.[25] However, in a recent multicenter randomized trial comparing FOLFIRI, m-IFL, and CapeIri in the first-line setting of mCRC, CapeIri was inferior in efficacy with a higher rate of toxicities compared to the other two regimens.[24]
Oxaliplatin
Oxaliplatin is a third-generation platinum compound with significant anticancer activity in CRC in combination with 5-FU. Its dose-limiting toxicity is chronic neuropathy, which is a dose-dependent sensory neuropathy that develops in up to 12% to 15% of patients when the cumulative dose is greater than 850 mg/m2.[4,28]
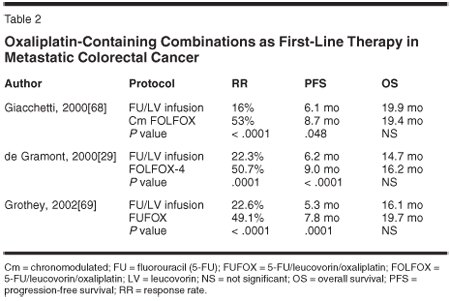
The benefit of adding oxaliplatin to 5-FU/LV was first reported in a randomized phase III trial by de Gramont et al.[29] The FOLFOX-4 regimen (oxaliplatin at a dose of 85 mg/m2 as a 2-hour infusion on day 1, every 2 weeks, plus LV5FU2) was compared with LV5FU2 alone in 420 patients with previously treated mCRC (Table 2). FOLFOX-4 resulted in a longer median progression-free survival (9 vs 6.2 months; P = .0003) and a higher response rate (50.7% vs 22.3%; P = .0001). The difference in median overall survival did not reach statistical significance (16.2 vs 14.7 months; P = .12). However, this trial was not sufficiently powered to detect such a difference and any potential survival difference may have been obscured as both treatment arms were able to receive salvage therapies. Grade 3/4 neutropenia and grade 3/4 diarrhea were more common with FOLFOX-4 but were at clearly manageable levels.[29]
FOLFOX-4 vs IFL
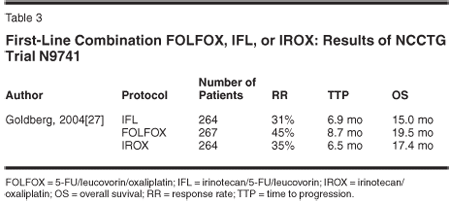
The Intergroup trial N9741 was a randomized phase III trial that compared FOLFOX, IFL, or oxaliplatin/irinotecan (IROX)[30] (Table 3). This pivotal trial showed that FOLFOX-4 was superior to IFL in RR (45% vs 31%; P = .002), TTP (8.7 vs 6.9 months; P = .0001), and median OS (19.5 vs 14.8 months; P = .0001). In addition, when compared to IFL or IROX, FOLFOX-4 was associated with a significantly lower incidence of febrile neutropenia and fewer gastrointestinal side effects. Based on this trial, FOLFOX-4 was approved in the United States as first-line treatment for patients with mCRC.[30]
FOLFIRI vs FOLFOX
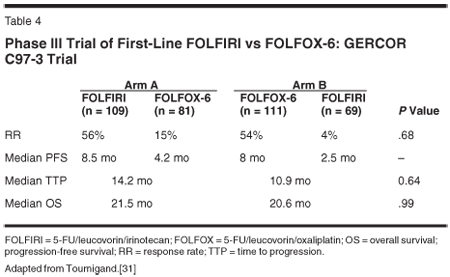
Tournigand et al conducted a randomized, multicenter, open-label prospective phase III trial in order to address the question of whether oxaliplatin is a more active agent than irinotecan when an identical 5-FU-based schedule is used.[31] Patients had equal access to the alternative regimen at progression. This trial used a simplified LV5FU2 regimen with a single 46-hour infusion. On one arm, patients received FOLFIRI (biweekly irinotecan at 180 mg/m2, LV at 200 mg/m2, 5-FU 400 mg/m2 on day 1 followed by a 46-hour continuous infusion of 5-FU at 2.4-3.0 g/m2) followed at progression by FOLFOX-6 (biweekly oxaliplatin at 100 mg/m2 and the same dose and schedule of 5-FU/LV), while patients in the second arm received the reverse sequence of FOLFOX-6 as first-line therapy followed by FOLFIRI at the time of progression.
The efficacy was similar in terms of RR (54% vs 56% for FOLFOX-6 and FOLFIRI, respectively), PFS (8 vs 8.5 months), and median OS (20.6 vs 21.5 months). Both treatment arms were relatively well-tolerated. Patients treated with first-line FOLFIRI experienced a higher incidence of grade 3/4 nausea and mucositis. The first-line FOLFOX-6 group experienced a higher incidence of grade 3/4 myelosuppression. The use of oxaliplatin was associated with grade 3/4 neurotoxicity (34%).
The Tournigand study was important as it demonstrated equivalent clinical efficacy between irinotecan and oxaliplatin in the first-line setting when used with the same de Gramont infusional 5-FU/LV regimen. It also demonstrated that there is not a superior sequence of two regimens (Table 4).[31] This conclusion was further supported by another randomized phase III trial that reported no difference in overall response rate, time to progression, and overall survival for mCRC patients treated with FOLFIRI or FOLFOX-4 as first-line regimens.[32]
Oxaliplatin Plus Capecitabine (CAPOX)
Capecitabine's convenience of being an oral agent with better tolerability than 5-FU/LV led to a series of studies that compared the combination of oxaliplatin with either capecitabine or 5-FU/LV. A phase II trial investigated the combination of capecitabine (1,000 mg/m2 twice daily from days 1 to 14) and oxaliplatin (130 mg/m2 IV on day 1) every 3 weeks. The regimen resulted in an overall response rate of 55% and a median overall survival of 19.5 months, comparable to that observed with FOLFOX-4.[33] This trial showed that the CAPOX combination is efficacious and well-tolerated, with common side effects being diarrhea, myelosuppression, and neurotoxicity. This result captivated the potential of capecitabine becoming an alternative to 5-FU/LV in the first-line setting.[33,34]
CAPIRI VS CAPOX and FUFOX VS CAPOX
Based on randomized trials data, CAPOX and a regimen consisting of weekly infusional 5-FU/LV/oxaliplatin (FUFOX) appeared to have similar antitumor activities, but CAPOX was associated with more significant gastrointestinal toxicities and myelosuppression.[35] A randomized phase II trial by Grothey et al compared the combination of capecitabine/irinotecan (CAPIRI) with CAPOX in the first line setting. Patients from both arms were allowed to cross over at progression. There were no differences in OS and the safety profiles of both regimens were equally manageable.[36] These trials further solidified the potential of capecitabine as an alternative to 5-FU/LV in the first-line setting.
Sequential vs Combination therapy
Grothey et al have shown that exposure to all three main anticancer agents during the treatment course of mCRC is a critical predictor of OS.[37] A few studies were conducted to assess whether sequential administration of these agents is superior to up-front combination therapy or vice versa. The Fluorouracil, Oxaliplatin, CPT-11 Usage Study (FOCUS) trial was a five-arm randomized phase III trial conducted to compare sequential therapy vs up-front use of combination regimens.[38] The up-front use of combination regimens such as FOLFOX or FOLFIRI yielded higher RR, PFS, and median OS. Initiation of combination therapy up-front would be reasonable in patients with good to excellent performance status, with aggressive disease, and potential candidates for salvage surgical resection. On the other hand, sequential therapy initiated with 5-FU/LV or capecitabine followed by FOLFOX or FOLFIRI as second-line options may be a reasonable approach in relatively asymptomatic patients with less aggressive and unresectable diseases.[38]
FOLFOXIRI
An approach of initiating combination therapy that contains all three cytotoxic agents (5-FU/LV, irinotecan, and oxaliplatin-FOLFOXIRI) together has been investigated. A phase III trial by Falcone et al compared FOLFOXIRI vs FOLFIRI in the first-line setting followed by an oxaliplatin-containing regimen as a second line. FOLFOXIRI was superior to FOLFIRI, yielding higher overall RR (66% vs 41%; P = .0002), median PFS (9.8 vs 6.9 months; P = .0006) and median OS (22.6 vs 16.7 months; P = .032). More patients in FOLFOXIRI went on to have resection of liver-only metastasis (36% vs 12%; P = .017). FOLFOXIRI was associated with higher incidence of grade 3/4 neuropathy and neutropenia.[39]
Souglakos et al conducted a similar trial but with lower FOLFOXIRI doses than Falcone's trial. This study reported no significant difference in RR, OS, or the ability to resect liver-only metastasis.[40] FOLFOXIRI may become a reasonable first-line option for a selective group of patients, particularly for potential candidates for definitive liver metastasis resections or for patients in whom biologic agents are contraindicated.
Optimal Duration of Chemotherapy
Maughan et al conducted a randomized study to show that by treating patients intermittently, toxicities were reduced without difference in overall survival vs continuous chemotherapy. This study provided evidence that discontinuing chemotherapy after a treatment period and reinitiating the same regimen at disease progression may be an appropriate option in chemosensitive disease.[41]
The OPTIMOX I trial compared FOLFOX-7 to FOLFOX-4. Patients in the FOLFOX-4 arm received the regimen continuously until disease progression, while patients treated with FOLFOX-7 received intermittent exposure to oxaliplatin allowing lower cumulative dose of neurotoxic oxaliplatin.[42] The FOLFOX-7 arm rendered a similar clinical efficacy with an improved safety profile when compared to the FOLFOX-4 arm. While this trial's outcome may have been obscured by the fact that there were a series of protocol violations in relation to the reintroduction of oxaliplatin, it was an important study suggesting that intermittent use of oxaliplatin-based chemotherapy may be a reasonable alternative with improved tolerability to continuous combination chemotherapy.[42]
The OPTIMOX II trial was a phase II study that evaluated a strategy involving a fully chemotherapy-free interval. In the investigational arm, patients received FOLFOX-7 with a modified reduced dose of oxaliplatin for six cycles followed by discontinuation of treatment. Patients were then reintroduced with modified FOLFOX-7 before the tumor reached its baseline measure. In the control arm, patients received modified FOLFOX-7 for six cycles followed by biweekly LV5FU maintenance therapy. Patients were reintroduced with modified FOLFOX-7 when there was disease progression to the baseline size of the tumor. The maintenance LV5FU arm showed longer PFS (8.7 vs 6.9 months; P = .09) However, there was no difference in the duration of disease control (12.9 vs 11.7 months; P = .41). In addition, the chemotherapy free interval was nearly 6 months.[43]
Another trial was conducted to evaluate whether the same efficacy of continuous chemotherapy can be achieved with intermittent chemotherapy. Labianca and colleagues compared FOLFIRI scheduled every 2 weeks continuously vs an alternating schedule of FOLFIRI for 2 months and a chemotherapy-free interval for 2 months. This trial reported no significant difference in PFS or median OS between the two treatment arms, supporting the suggestion that intermittent chemotherapy may be a potential alternative to continuous chemotherapy.[44] A chemotherapy-free interval without significant compromise of progression-free and overall survival can potentially have significant impact on the quality of life of metastatic colorectal cancer patients.
Novel Targeted Agents
Bevacizumab
Bevacizumab is a recombinant humanized monoclonal antibody targeted against vascular endothelial growth factor (VEGF), which is a proangiogenic growth factor that is overexpressed in CRC as well as in a wide range of solid human cancers.[4] Bevacizumab monotherapy does not appear to have significant activity in mCRC. On the other hand, it has considerable activity when used in combination with commonly used chemotherapies, including 5-FU, irinotecan, oxaliplatin, and possibly in combination with cetuximab.[3,45,46]
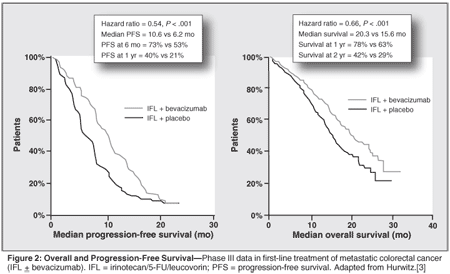
Hurwitz et al reported phase III trial data showing that the addition of bevacizumab to IFL led to statistically significant improvement of OS (20.3 vs 15.6 months; P = .001), RR (44.8% vs 34.8%; P = .004), and PFS (10.6 vs 6.2 months) in patients with mCRC (Figure 2). Patients who progressed after IFL plus bevacizumab then went on to receive second-line therapy with an oxaliplatin-based regimen and achieved an overall survival of 25.1 months, which was the longest median overall survival rendered by any first- and second-line combination therapy at the time.[3] One can reasonably presume that an even better outcome might have resulted by using the infusional 5-FU/LV schedule (FOLFIRI), which now is a standard of care, rather than the bolus 5-FU/LV schedule (IFL).
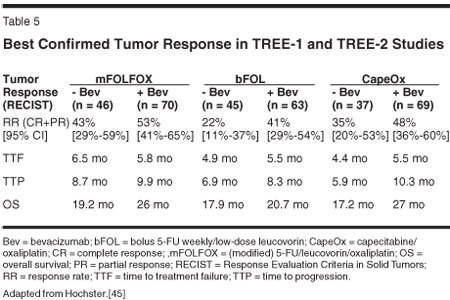
The final analysis of the TREE-1 and -2 trials reported that the addition of bevacizumab to bolus, infusional, or capecitabine regimen rendered improved response rates in all groups. This important trial showed that FOLFOX plus bevacizumab treatment was a superior first-line regimen to FOLFOX alone, improving overall RR (43% vs 53%), median TTP (8.7 vs. 9.9 months), and median OS (19.2 vs 26 months) (Table 5). Bevacizumab is now recommended to be used with all five first-line therapy regimens outlined by the NCCN.[7,45]
The Eastern Cooperative Oncology Group (ECOG) 3200 study, including 820 patients who were previously treated with first-line 5-FU/irinotecan either separately or as a combination regimen without bevacizumab, reported that adding bevacizumab to the second-line FOLFOX-4 regimen improved median overall survival when compared to FOLFOX-4 alone (12.5 vs 10.7 months; P = .002).[46]
Bevacizumab as third-line therapy appears to have very limited activity in patients who failed previous treatment with oxaliplatin- or irinotecan-based regimens.[47] The optimal duration of bevacizumab, particularly in patients who progressed on first-line combination therapy with bevacizumab, is unclear at this time. The Southwest Oncology Group is conducting a phase II trial in an attempt to answer this question.
Bevacizumab is generally well-tolerated and has very few overlapping toxicities with chemotherapeutic agents. Hurwitz et al reported grade 3 hypertension (11%) that was managed with oral medications and an increased risk of gastrointestinal perforation (1.5%). However, there was no significant difference in treatment-related hospitalization, treatment delay due to toxicity, or the 60-day rate of death of all cause.[3] Skillings et al reviewed 1,745 patients who received bevacizumab and identified approximately twofold increased risk of arterial thromboembolic events. Bevacizumab was also associated with bleeding (2%-9.3%), proteinuria (1%-2%), and poor wound healing (1%-2%).[48] These side effects should be considered when evaluating patients for the use of bevacizumab. The recommended dose of bevacizumab in treating first-line mCRC is 5 mg/kg IV administered over 90 minutes, while 10 mg/kg IV is the dose for second-line treatment. A dose of 7.5 mg/kg IV every 3 weeks can be used when bevacizumab is used with capecitabine-containing regimens.[3,4,49]
Cetuximab
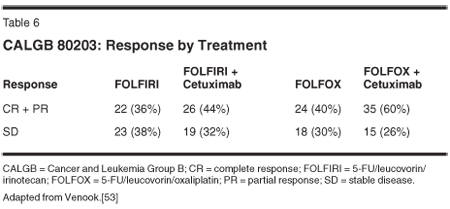
Cetuximab is a recombinant human/mouse chimeric epidermal growth factor receptor (EGFR) monoclonal antibody. EGFR is overexpressed in CRC as well as in a broad range of solid tumors. Preliminary data on cetuximab in combination with FOLFIRI, FOLFOX, and XELOX first-line is encouraging, and this area is under further investigation[4,50-55] (Table 6).
Currently, cetuximab is a recommended second-line agent in mCRC patients who cannot tolerate, progressed on, or failed irinotecan-based chemotherapy.[7] In the BOND-1 trial, the combination of cetuximab with irinotecan was found to be superior to cetuximab alone in patients who progressed on an irinotecan-based first-line regimen. Cetuximab is also recommended as a third-line therapy for patients who failed irinotecan- or oxaliplatin-based chemotherapies. Cetuximab was associated with acneiform rash (up to 88% of all patients), severe infusion reaction (3%), interstitial lung disease, and hypomagnesaemia. Cetuximab is administered with a loading dose of 400 mg/m2 IV over 90 minutes, followed by a maintenance dose of 200 mg/m2 IV given weekly.[4,7,56]
Panitumumab
Panitumumab is a human monoclonal antibody targeting the epidermal growth factor receptor. Panitumumab monotherapy has recently been approved by the FDA based on clinical trials that showed activity in mCRC patients with prior chemotherapy.[57-62]
Gibson et al conducted a randomized phase III trial that compared panitumumab as a single agent to best supportive care in patients previously treated with mCRC. In this study, panitumumab was shown to be efficacious (46% reduction in the risk of tumor progression and a partial response rate of 8%) with a manageable side-effect profile.[60]
The use of panitumumab in combination with other cytotoxic agents in first-line setting appears to be an option that is worthy of further investigation. In a phase II trial, the combination of IFL and panitumumab as first-line therapy showed a disease control rate (OR + SD) of 74% with a median PFS of 5.6 months and an OS of 17 months. In this trial, panitumumab was also combined with FOLFIRI and resulted in similar efficacy with a disease control rate of 79% (33% PR and 46% SD) and a median PFS of 10.9 months. The survival data is still maturing. In terms of side effects, fewer patients experienced grade 3/4 diarrhea (25% vs 47%). Skin-related toxicities were reported up to 17% in the FOLFIRI group and 16% in the IFL group.[63,64]
A randomized phase III trial (PACCE) is being conducted to evaluate the possible role of panitumumab when used in combination with currently available regimens such as FOLFOX plus bevacizumab and FOLFIRI plus bevacizumab.[64]
Vatalanib
Vatalanib is an oral small-molecule VEGF receptor inhibitor that blocks the activity VEGF receptor tyrosine kinase. Preliminary results from CONFIRM-1 study by Hecht et al suggest improved outcome with FOLFOX by adding vatalanib in treating chemotherapy-naive patients with mCRC.[65] The initial primary end point of the study, which was PFS, showed only a modest statistically insignificant benefit for adding vatalanib to FOLFOX-4. However, a subsequent analysis showed a statistically significant improvement in PFS with vatalanib in patients with elevated pretreatment lactate dehydrogenase. Final survival data on this trial are maturing. Common side effects of vatalanib were hypertension (21%), neutropenia (31%), diarrhea (15%), nausea (9%), peripheral neuropathy (9%), venous thrombosis (7%), dizziness (7%), and pulmonary embolism (6%).[65,66]
Combination of Targeted Agents
The BOND-2 trial demonstrated that, in irinotecan-resistant mCRC, combining bevacizumab/cetuximab with irinotecan improved outcomes compared to bevacizumab/cetuximab alone.[67] This trial suggested that adding bevacizumab to a cetuximab-containing regimen may improve the outcome. Active investigations are in progress to define the role of combinations of targeted agents in first-line as well as second-line settings.
Disclosures:
Dr. Chu has acted as a consultant for and received research support from Roche, Pfizer, Bristol-Myers Squibb/ImClone, and Genentech. Dr. Saif has received honoraria for serving on speakers bureaus for Genentech, Sanofi-Aventis, BristolMyers-Squibb, Amgen, Pfizer, Roche, and Novartis.
References:
1. American Cancer Society. Cancer Facts & Figures 2006. Available at http://www.cancer.org.
2. Meta-Analysis Group in Cancer: Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: An updated meta-analysis. J Clin Oncol 22:3766-3775, 2004.
3. Hurwitz H, Fehrenbacher L, Novotny W, et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335-2342, 2004.
3a. Grem JL: 5-Fluorouracil: Forty-plus and still ticking. A review of its clinical development. Invest New Drugs 18(4):299-313, 2000.
4. Chu E, Devita V: Physicians' Cancer Chemotherapy Drug Manual. Jones & Bartlett, 2006.
5. Meyerhardt JA, Mayer RJ: Systemic therapy for colorectal cancer. N Eng J Med 352:476-487, 2005.
6. Poon MA, O'Connell MJ, Moertel CG, et al: Biochemical modulation of fluorouracil: Evidence of significant improvement of survival and quality of life in patents with advanced colorectal carcinoma. J Clin Oncol 7:1407-1418, 1989.
6. Hoff P, Pazdur R: Progress in the development of novel treatments for colorectal cancer. Oncology 18:705-708, 2004.
7. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Colon Cancer. Version 2.2006. Available at http://nccn.org/professionals/physician_gls/PDF/colon.pdf
8. Buroker TR, O'Connell MJ, Wieand HS, et al: Randomized comparison of two schedules of fluorouracil and leucovorin in the treatment of advanced colorectal cancer. J Clin Oncol 12:14, 1994.
9. Wang WS, Lin JK, Chiou TJ, et al: Randomized trial comparing weekly bolus 5-fluorouracil plus leucovorin versus monthly 5-day 5-fluororuacil plus leucovorin in metastatic colorectal cancer. Hepatogastroenterology 47:1599, 2000.
10. The Meta-Analysis Group in Cancer: Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol 16:301-308, 1998.
11. de Gramont A, Bosset JF, Milan C, et al: Randomized trial comparing monthly low dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: A French Intergroup study.
J Clin Oncol 15:808, 1997.
12. The Meta-Analysis Group in Cancer: Toxicity of fluorouracil in patients with advanced colorectal cancer: Effect of administration schedule and prognostic factors. J Clin Oncol 11:3537-3541, 1998.
13. Weh HJ, Wilke HJ, Dierlamm J, et al: Weekly therapy with folinic acid (FA) and high-dose fluorouracil (5-FU) 24 h infusion in pretreated patients with metastatic colorectal carcinoma. A multicenter study by the Association of Medical Oncology of the German Cancer Society (AIO). Ann Oncol 5:233-237, 1994.
14. Chu E, Eng C, Abbruzzese J, et al: Efficacy and safety of capecitabine for colorectal cancer. Am J OncolRev 2(suppl 3):1-28, 2003.
15. Schuller J, Cassidy J, Dumont E, et al: Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol 45:291, 2000.
16. Hoff PM, Ansari R, Batist G, et al: Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: Results of a randomized phase III study. J Clin Oncol 19:2282-2292, 2001.
17. Van Cutsem E, Twelves C, Cassidy J, et al: Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: Results of a large phase III study. J Clin Oncol 19:4097-4106, 2001.
18. Saif MW: Capecitabine versus continuous-infusion 5-fluorouracil for colorectal cancer. A retrospective efficacy and safety comparison. Clin Colorectal Cancer 5(2):89-100, 2005.
19. Vanhoefer U, Harstrick A, Achterrath W, et al: Irinotecan in the treatment of colorectal cancer: Clinical overview. J Clin Oncol 19:1501-1518, 2001.
20. Cunningham D, Pyrhonen S, James RD, et al: Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 352:1413, 1998.
21. Douillard JY, Cunningham D, Roth AD, et al: Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicenter randomised trial. Lancet 355:1041, 2000.
22. Kohne CH, van Cutsem E, Wils J, et al: Phase III study of weekly high-dose infusional fluorouracil plus folinic acid with or without irinotecan in patients with metastatic colorectal cancer: European Organization for Research and Treatment of Cancer Gastrointestinal Group Study 40986. J Clin Oncol 23:4856, 2005.
23. Saltz LB, Cox JV, Blanke C, et al: Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905-914, 2000.
24. Fuchs C, Marshall J, Mitchell E, et al: A randomized trial of first-line irinotecan/fluoropyrimidine combinations with or without celecoxib in metastatic colorectal cancer (BICC-C) (abstract 3506). J Clin Oncol 24(suppl 18S):147s, 2006.
25. Ahn Kh, Jung YS, Park YH, et al: Phase II trial of irinotecan and capecitabine in patients with advanced colorectal cancer (abstract 3714). Proc Am Soc Clin Oncol 23:299s, 2005.
26. Patt YZ, Leibmann J, Diamondidis D, et al: Capecitabine plus irinotecan as first-line treatment for metastatic colorectal cancer: Final safety findings from a phase II trial (abstract). Proc Am Soc Clin Oncol 23:271a, 2004.
27. Bajetta E, Di Bartolomeo M, Mariani L, et al: Randomized multicenter Phase II trial of two different schedules of irinotecan combined with capecitabine as first-line treatment in metastatic colorectal carcinoma. Cancer 100:279, 2004.
28. Grothey A, Goldberg RM: A review of oxaliplatin and its clinical use in colorectal cancer. Expert Opin Pharmacother 5:2159-2170, 2004.
29. de Gramont A, Figer A, Seymour M, et al: Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938, 2000.
30. Goldberg RM, Sargent DJ, Morton RF, et al: A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23, 2004.
31. Tournigand C, Andre T, Achille E, et al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol 22:229-237, 2004.
32. Colucci G, Gebbia V, Paoletti G, et al: Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol 23(22):4866-4875, 2005.
33. Cassidy J, Tabernero J, Twelves C, et al: XELOX (capecitabine plus oxaliplatin): Active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol 22:2084, 2004.
34. Scheithauer W, Kornek GV, Raderer M, et al: Randomized multicenter phase II trial of two different schedules of capecitabine plus oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 21:1307-1312, 2003.
35. Arkenau H, Schmoll H, Kubicka S, et al: Infusional 5-fluorouracil/folinic acid plus oxaliplatin (FUFOX) versus capecitabine plus oxaliplatin (CAPOX) as first line treatment of metastatic colorectal cancer (MCRC): Results of the safety and efficacy analysis (abstract 3507). J Clin Oncol 23:247s, 2005.
36. Grothey A, Jordan K, Kellner O, et al: Capecitabine/irinotecan (CapIri) and cape-citabine/oxaliplatin (CapOx) are active second-line protocols in patients with advanced colorectal cancer after failure of first-line combination therapy: Results of a randomized phase II study (abstract 3534). Proc Am Soc Clin Oncol 22:14s 2004.
37. Grothey A, Sargent D, Goldberg RM, et al: Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22:1209-1214, 2004.
38. Maughan T, on behalf of the NCRI Colorectal Group: Fluorouracil, oxaliplatin, CPT-11(irinotecan), use and sequencing, in advanced colorectal cancer: the UK MRC FOCUS Trial (abstract 165). J Clin Oncol 23(suppl 18S):2005.
39. Falcone A, Masi G, Murr R, et al: Bi-weekly irinotecan, oxaliplatin, and infusion-al 5FU/LV (FOLFOXIRI) versus FOLFIRI as first-line treatment of metastatic colorec-tal cancer (MCRC): Results of a randomized, phase III trial by the GONO (abstract 227). Proceedings of the 2006 Gastrointestinal Cancer Symposium, January 26-28, 2006, San Francisco.
40. Souglakos J, Androulakis N, Syrigos K, et al: FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs. FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): A multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). Br J Cancer 94:798-805, 2006.
41. Maughan TS, Kerr J, Ledermann M, et al: Comparison of intermittent and continuous palliative chemotherapy for advanced colorectal cancer: A multicenter randomized trial. Lancet 361:457-464, 2003.
42. de Gramont A, Cervantes A, Andre T, et al: OPTIMOX study: FOLFOX/LV5FU2 compared to FOLFOX 4 in patients with advanced colorectal cancer (abstract 3525). Proc Am Soc Clin Oncol 23:251, 2004.
43. F. Maindrault-Goebel, G. Lledo, Chibaudel B, et al: OPTIMOX2, a large randomized phase II study of maintenance therapy or chemotherapy-free intervals (CFI) after FOLFOX in patients with metastatic colorectal cancer (MRC). A GERCOR study (abstract 3504 ). J Clin Oncol 24(suppl 18S):147s, 2006.
44. Labianca R, Floriani I, Cortesi E, et al: Alternating versus continuous "FOLFIRI" in advanced colorectal cancer (ACC): A randomized "GISCAD" trial (abstract 3505). J Clin Oncol 24(suppl 18S):147s, 2006.
45. Hochster HS, Welles L, et al: Safety and efficacy of bevacizumab when added to oxaliplatin/fluoropyrimidine regimens as first line treatement of metastatic colorectal cancer: TREE 1&2 study (abstract 3515). J Clin Oncol 23(suppl 16S):249s, 2005.
46. Giantonio BJ, Catalano G, Meropol NJ, et al: High-dose bevacizumab improves survival when combined with FOLFOX4 in previously treated advanced colorectal cancer: Results from the ECOG study E3200 (abstract 2). J Clin Oncol 23(suppl 16S):1s, 2005.
47. Chen HX, Mooney M, Boron M, et al: Bevacizumab plus 5-FU/leucovorin for advanced colorectal cancer that progressed after standard chemotherapies: An NCI Treatment Referral Cancer Trial (abstract 3515). Proc Am Soc Clin Oncol 23:249a, 2004.
48. Saif MW, Mehra R: Incidence and management of bevacizumab-related toxicities in colorectal cancer. Expert Opin Drug Saf 5(4):553-566, 2006.
49. Skillings JR, Johnson DH, Miller K, et al. Arterial thromboembolic events in a pooled analysis of 5 randomized, controlled trials of bevacizumab with chemotherapy (abstract 3019). J Clin Oncol 23(suppl 16S):196s, 2005.
50. Rougier P, Raoul JL, van Laethem J-L, et al: Cetuximab + FOLFIRI as first line treatment for metastatic colorectal CA (abstract 3513). Proc Am Soc Clin Oncol 23:248, 2004.
51. Diaz-Rubio E, Tabernero J, Van Cutsem E, et al: Cetuximab in combination with oxaliplatin, 5-FU/LV (FOLFOX-4) in the first line treatment of patients with epidermal growth factor receptor-expressing metastatic colorectal cancer: an international phase II study (abstract 3535). J Clin Oncol 23(suppl 16S):254s, 2005.
52. Borner M, Mingrone W, Koeberle D, et al: The impact of cetuximab on the capecitabine plus oxaliplatin (XELOX) combination in first-line treatment of metastatic colorectal cancer: A randomized phase II trial of the Swiss Group for Clinical Cancer Research (abstract 3551). J Clin Oncol 24(suppl 18S):158s, 2006.
53. Venook A, Niedzwiecki D, Hollis D, et al: Phase III study of irinotecan/5FU/LV (FOLFIRI) or oxaliplatin/5FU/LV (FOLFOX) ± cetuximab for patients (pts) with untreated metastatic adenocarcinoma of the colon or rectum (MCRC): CALGB 80203 preliminary results (abstract 3509). J Clin Oncol 24(suppl 18S):148s, 2006.
54. Dakhil S, Cosgriff T, Headley D, et al: Cetuximab + FOLFOX6 as first line therapy for metastatic colorectal cancer (An International Oncology Network study, I-03-002) (abstract 3557). J Clin Oncol 24(suppl 18S):160s, 2006.
55. Heinemann V, Fischer Von Weikersthal L, Moosmann N, et al: Cetuximab + capecitabine + irinotecan (CCI) versus cetuximab + capecitabine + oxaliplatin (CCO) as first line therapy for patients with metastatic colorectal cancer (CRC): Preliminary results of a randomized phase II trial of the AIO CRC Study Group (abstract 3550). J Clin Oncol 24(suppl 18S):158s, 2006.
56. Cunningham D, Humblet Y, Siena S, et al: Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337-345, 2004.
57. Yang XD, Xiao-Chi J, Corvalan JRF, et al: Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer chemotherapy. Crit Rev Oncol Hematol 38:17-23, 2001.
58. Lynch DH, Yang XD: Therapeutic potential of ABX-EGF: A fully human anti-epidermal growth factor receptor monoclonal antibody for cancer treatment. Semin Oncol 29(suppl 4):47-50, 2002.
59. Malik I, Hecht JR, Patnaik A, et al: Safety and efficacy of panitumumab monotherapy in patients with metastatic colorectal cancer (abstract 3520). J Clin Oncol 23(suppl 16S):251s, 2005.
60. Gibson TB, Ranganathan A, Grothey A, et al: Randomized phase III trial results of panitumumab, a fully human anti-epidermal growth factor receptor monoclonal antibody, in metastatic colorectal cancer. Clin Colorectal Cancer 6(1):29-31, 2006.
61. Saif MW, Cohenuram M: Role of panitumumab in the management of metastatic colorectal cancer. Clin Colorectal Cancer 6(2):118-124, 2006
62. Hecht JR, Patnaik A, Malik I, et al: ABX-EGF Monotherapy in patients with metastatic colorectal cancer: An updated analysis (abstract 3511). Proc Am Soc Clin Oncol 23:248, 2004
63. Berlin J, Malik I, Picus J: Panitumumab therapy with irinotecan, 5-fluorouracil, and leucovorin (IFL) in metastatic colorectal patients (abstract 265PD). Proc Eur Soc Med Onc 2004.
64. PACCE: A randomized, open-label, controlled, clinical trial of chemotherapy and bevacizumab with and without panitumumab in the first-line treatment of subjects with metastatic colorectal cancer. National Cancer Institute website. Available at www.clinicaltrials.
gov.ct/show/NCT00115765
65. Hecht JR, Trarbach T, Jaeger E, et al: A randomized, double-blind, placebo-controlled, phase III study in patients with metastatic adenocarcinoma of the colon or rectum receiving first-line chemotherapy with oxaliplatin/5-FU/LV, and PTK 787/ZK222584 or placebo (CONFIRM-1) (abstract LBA3). J Clin Oncol 23(suppl 16S):2005.
66. Alessandro M, et al: Combination targeted therapy tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: Current status and future directions. Oncologist 11(7):753-764, 2006.
67. Saltz LB, Lenz H, Hochster H, et al: Randomized phase II trial of cetuximab/bevacizumab/irinotecan (CBI) versus cetux-imab/bevacizumab (CB) in irinotecan-
refractory colorectal cancer (abstract 3508). J Clin Oncol 23(suppl 16S):248s, 2005.
68. Giacchetti S, Perpoint B, Zidani R, et al: Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluoro-uracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 18(1):136-147, 2000.
69. Grothey A, Deschler B, Kroening H, et al: Phase III study of bolus 5-fluorouracil (5-FU)/folinic acid (FA) (Mayo) vs weekly high-dose 24h 5-FU infusion/FA + oxaliplatin in advanced colorectal cancer (abstract 512). Proc Am Soc Clin Oncol 21:129a, 2002.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.