Reduced-Dose Capecitabine May Be Reasonable Alternative to Combination Chemotherapy
OTTAWA, CANADA-Forolder and less fit cancer patients, whoare more likely to experience greatertoxicity but less benefit from combinationchemotherapy, a more reason
OTTAWA, CANADA-Forolder and less fit cancer patients, whoare more likely to experience greatertoxicity but less benefit from combinationchemotherapy, a more reasonablealternative might be single-agentand reduced-dose chemotherapy. Thatwas the rationale behind a Canadianphase I/II trial to assess reduced-dosecapecitabine (Xeloda) as part of a sequentialsingle-agent therapy for olderand less fit patients with advancedcolorectal cancer (abstract 3577).The data were presented by M. C.Cripps, MD, of the Ottawa RegionalCancer Center.Results showed an overall mediansurvival of 14.8 months and a "respectable19.7 month median survivalin older patients" with an Eastern CooperativeOncology Group performancestatus (ECOG PS) of 0, theinvestigators reported. This finding"suggests sequential single-agent therapybeginning with capecitabine maybe a reasonable and well-tolerated alternativeto combination chemotherapy,"the investigators concluded.Five Overlapping SubgroupsThe multicenter trial was designedto test reduced-dose capecitabine in apopulation of adults with advancedcolorectal cancer "clearly shown notto derive survival benefit from combinationchemotherapy." Study participantscould have undergone no priorchemotherapy for metastatic disease,and adjuvant fluorouracil therapymust have been completed 6 or moremonths before study enrollment.The male/female ratio for the 221patients accrued was 142/79, or 64%/36%. Patients fell into one or more ofthe following five subgroups at baseline:
- Age ≥ 65 years (173 patients[78%])
- ECOG PS ≥ 1 (145 patients[66%])
- Liver enzyme abnormalities (5patients [2%]).
- Prior pelvic radiation therapy(54 patients [24%])
- Liver enzyme abnormalities (5patients [2%]).
The number of patients with abnormalliver function tests was consideredinsufficient to move this subgroupinto the phase II stage to evaluatefor toxicity.
Capecitabine was administered at1,000 mg/m
2
twice daily on days 1 to14 every 21 days. The median numberof cycles was five. Prior pelvic radiationtherapy required dose reductionto 750 mg/m
2
twice daily due to diarrheain the third cycle.
Factors Predicting Survival
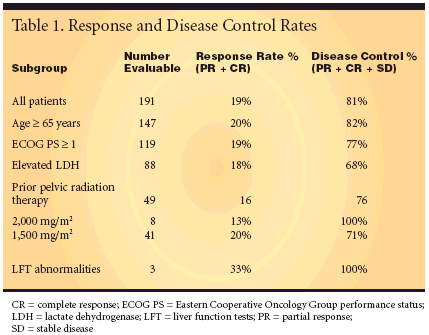
Among the 191 evaluable patients,the overall response rate (completeplus partial responses) was 19%, andthe disease control rate (overall responseplus stable disease) was 81%.Disease control rates were somewhatlower for those patients with poorerECOG PS, an elevated LDH level, andhigher doses of pelvic radiation therapy(see Table 1).The median disease progressionfreesurvival was 5.2 months (95%confidence interval [CI], 4.5-6.9). Theoverall median survival at the time ofthis report was 14.8 months (95% CI,11.8-17.0). At median follow-up of11.1 months, 82 patients were still alive.Factors predicting shorter survivalwere poor ECOG PS and elevated LDHlevel. Although the median survivaltime for patients with an ECOG PS of0 was 19.7 months, it fell to 13.2 monthsfor patients with an ECOG PS of 1 and6.8 months for patients with an ECOGPS of 2.
Grade 3/4 Adverse Events
Of the 213 patients evaluable fortoxicity, 23 had grade 3/4 adverseevents. The two most common adverseevents were hand-foot syndrome,experienced by eight patients,and diarrhea, experienced by seven.Other Canadian institutions participatingin the trial were the LondonRegional Cancer Centre, British ColumbiaCommunity Oncology Associatesin Vancouver, the British ColumbiaCancer Agency in Kelowna,the Windsor Regional Cancer Centre,the Kingston Regional Cancer Centre,and the Grand River Regional CancerCentre in Kitchener.