Risk Models for Chemotherapy-Induced Neutropenia in Non-Hodgkin’s Lymphoma
Non-Hodgkin’s lymphoma is primarily a disease of the elderly, with61% of the new cases reported in patients 60 years old or older. Aggressivecombination chemotherapy can cure some patients, but there arefrequently treatment failures and overall survival is low. Retrospectivestudies have found that treatment with less than standard chemotherapydoses is associated with lower survival, and surveys of practice patternshave found that many patients, especially elderly ones, are treated withsubstandard regimens and doses. Neutropenia is the major dose-limitingtoxicity of chemotherapy in patients with non-Hodgkin’s lymphoma.First-cycle use of colony-stimulating factor (CSF) can reduce the incidenceof neutropenia and its complications and help maintain the chemotherapydoses. Researchers have investigated risk factors in patientswith non-Hodgkin’s lymphoma to determine which patients are at highestrisk for neutropenia and would benefit from targeted first-cycle CSFsupport. It has been shown in several studies that advanced age, poorperformance status, and high chemotherapy dose intensity are risk factors.Other trials suggest that low serum albumin levels, elevated lactatedehydrogenase levels, bone marrow involvement, and high levelsof soluble tumor necrosis factor receptor are also risk factors. Doseintensity has also been shown in many studies to be an important predictorof survival in patients with non-Hodgkin’s lymphoma. Managingthe toxicity of chemotherapy with CSF has facilitated the deliveryof planned dose on time, as well as dose-intensified chemotherapy regimens.The promising results from recent clinical trials of dose-denseregimens with CSF support suggest that this could prove to be the beststrategy for improving patient outcomes.
ABSTRACT: Non-Hodgkin’s lymphoma is primarily a disease of the elderly, with61% of the new cases reported in patients 60 years old or older. Aggressivecombination chemotherapy can cure some patients, but there arefrequently treatment failures and overall survival is low. Retrospectivestudies have found that treatment with less than standard chemotherapydoses is associated with lower survival, and surveys of practice patternshave found that many patients, especially elderly ones, are treated withsubstandard regimens and doses. Neutropenia is the major dose-limitingtoxicity of chemotherapy in patients with non-Hodgkin’s lymphoma.First-cycle use of colony-stimulating factor (CSF) can reduce the incidenceof neutropenia and its complications and help maintain the chemotherapydoses. Researchers have investigated risk factors in patientswith non-Hodgkin’s lymphoma to determine which patients are at highestrisk for neutropenia and would benefit from targeted first-cycle CSFsupport. It has been shown in several studies that advanced age, poorperformance status, and high chemotherapy dose intensity are risk factors.Other trials suggest that low serum albumin levels, elevated lactatedehydrogenase levels, bone marrow involvement, and high levelsof soluble tumor necrosis factor receptor are also risk factors. Doseintensity has also been shown in many studies to be an important predictorof survival in patients with non-Hodgkin’s lymphoma. Managingthe toxicity of chemotherapy with CSF has facilitated the deliveryof planned dose on time, as well as dose-intensified chemotherapy regimens.The promising results from recent clinical trials of dose-denseregimens with CSF support suggest that this could prove to be the beststrategy for improving patient outcomes.
The incidence of and mortalityin 8 of the 10 most commontypes of cancer in the UnitedStates are decreasing.[1,2] One ofthose in which they are increasing isnon-Hodgkin's lymphoma,[3] and it isestimated that 53,400 new cases ofnon-Hodgkin's lymphoma and 23,400deaths due to it will occur in the UnitedStates in 2003.[4] These increaseshave been most notable in patientsolder than 60 years,[5] in whom 61%of the new cases of the disease occur.Combination chemotherapy withCHOP (cyclophosphamide [Cytoxan,Neosar], doxorubicin HCl, vincristine[Oncovin], prednisone), CHOP-like,or CHOP-R (plus rituximab [Rituxan])regimens is considered the standard ofcare in young and older patients alikewith aggressive non-Hodgkin's lymphoma.[6-8] Non-Hodgkin's lymphomais potentially curable and completeresponses are achieved in manypatients, but treatment failures andmortality are more common in olderpatients.[6] In fact, age 60 years andolder is a prognostic factor for adverseoutcomes in patients with intermediate-grade non-Hodgkin's lymphoma.[9] The CHOP regimen producescomplete responses in only 40%to 50% of elderly patients, with 3-yearevent-free and overall survival being30% and 35% to 40%, respectively.[10] Nonmyeloablative, doseintensifiedchemotherapy regimensmay improve the outcomes in youngerpatients, but they are generally notwell tolerated in elderly patients. Thelower survival in elderly patients hasbeen attributed to the greater toxicityof standard full-dose chemotherapy inthe presence of comorbidities and poorbone marrow reserves.[11]
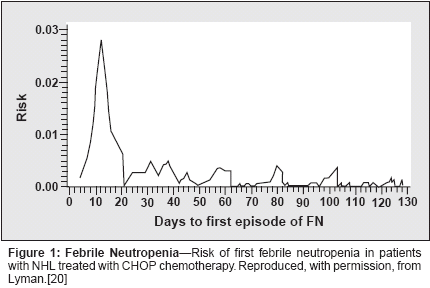
Concern over elderly patients' toleranceof chemotherapy has led manyphysicians to treat them less aggressively.This may be accomplishedeither by administering lessmyelosuppressive(and potentiallyless-effective) chemotherapy or byadministering substandard dose intensities.A retrospective analysis of datafrom a large survey of practice patternsin patients with non-Hodgkin's lymphomafound that older patients weresignificantly more likely than youngerpatients to be treated with a plannedaverage relative dose intensity of 80%or less (28% vs 12%, P < .001).[12]Elderly patients were also more likelyto be treated with less-aggressive, andtherefore potentially less-effective,regimens than younger patients. Forexample, non-anthracycline-containingregimens such as CNOP (cyclophosphamide,mitoxantrone [Novantrone],vincristine, prednisone) andCVP (cyclophosphamide, vincristine,prednisone) were more commonlyused in patients aged 60 years andolder than in younger patients (21%vs 7%, P < .001).This practice may be one of the reasonsfor the lower survival rates in theelderly. Routine undertreatment maybe due to assumptions about theirfrailty or relative inability to toleratechemotherapy. However, there is considerableevidence that elderly patientswill benefit as much as younger patientswhen they are given equivalentchemotherapy doses. A classic exampleof this is seen in the results of astudy by the Southwest OncologyGroup (SWOG) in 307 patients withlarge cell lymphoma treated withCHOP.[13] Of the 81 elderly patientsin the study, 23 were given full-dosetreatment in violation of the protocolstipulated50% reduction in the chemotherapydose in patients aged 65years or older. Analysis of the resultsin these 23 patients found no significantdifferences between them andyounger patients in the complete responserate, duration of complete response,or frequency of treatment-relatedcomplications. The investigatorstherefore concluded that the routinereduction in chemotherapy doses onthe basis of age alone is inappropriateand may be a primary contributor tothe poorer outcomes in elderly patients.In light of the results of this andother studies in non-Hodgkin's lymphoma,the relation between dose intensityand outcomes in this diseasehas become well established. Severalretrospective studies have shown thatmaintaining the standard dose intensityof the chemotherapy increases thelikelihood of a complete response andgreater survival.[14-16] Kwak andcolleagues reported that the strongestfactor associated with greater survivalwas the delivery of more than 75% ofthe relative dose intensity of doxorubicin.[14] Similarly, Epelbaum andcolleagues found that delivery of anaverage relative dose intensity of morethan 80% resulted in a significantlyhigher complete response rate and thatthe delivery of more than 70% resultedin higher 5-year overall survival.[15]Lepage and colleagues found that theresponse rate and overall survival weresignificantly lower in patients withaggressive non-Hodgkin's lymphomawho were treated with less than 70%of the planned dose intensity.[16]Older patients were shown to haveoutcomes similar to those in youngerpatients when the dose intensity wasmaintained with the use of CSF.[17]It is clear that maintaining the doseintensity of the chemotherapy is importantin non-Hodgkin's lymphoma,and managing dose-limiting toxicitiesplays an important role in this.Chemotherapy-InducedNeutropeniaSevere neutropenia and its complicationsare the major dose-limitingtoxicities of chemotherapy for non-Hodgkin's lymphoma. Hospitalizationfor febrile neutropenia is associatedwith substantial costs, lower quality oflife, and a greater risk of mortality,especially in elderly patients withcomorbidities. In a study that compareddata on hospitalizations for febrileneutropenia in patients with lymphomaand patients with solid tumors,the mean lengths of stay were longer(10.8 vs 8.5 days), the costs per episodegreater ($19,061 vs $12,302),and treatment-related mortality higher(10% vs 9%) in patients with lymphoma.[18] The complications of neutropeniain patients with lymphomamay lead to treatment delays and dosereductions, seriously compromisingthe dose intensity in patients with thispotentially curable cancer. As wasnoted above, the risk of neutropeniais a major factor in the choice of chemotherapyand in regimen modifications,and this can result in suboptimaltreatment.The first occurrence of febrile neutropeniais most likely in the earlycycles of chemotherapy. A randomizedtrial that assessed the toxicity of chemotherapyin 453 elderly patients withnon-Hodgkin's lymphoma reportedthat 43% to 68% of all occurrences ofgrades 3 and 4 leukopenia were in thefirst cycle, depending on the chemotherapy regimen.[19] A retrospectiveanalysis of data on 577 patients withnon-Hodgkin's lymphoma found that62% of the initial occurrences of febrileneutropenia were in the first cycle(Figure 1).[20] Another study foundthat more than 65% of the hospitalizationsfor febrile neutropenia werein cycles 1 and 2.[21] Furthermore, aretrospective analysis of the data on267 elderly patients treated withCHOP found that 63% of the treatment-related deaths occurred in thefirst cycle.[22] These findings make itclear that neutropenia and its complicationsare best prevented early in thecourse of chemotherapy.
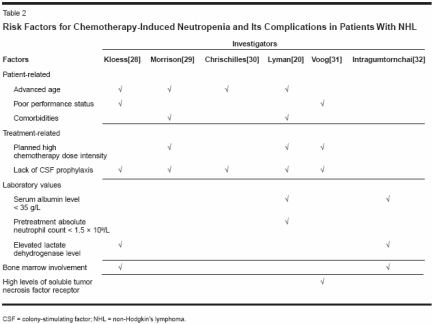
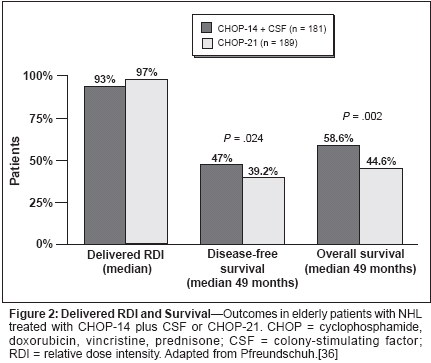
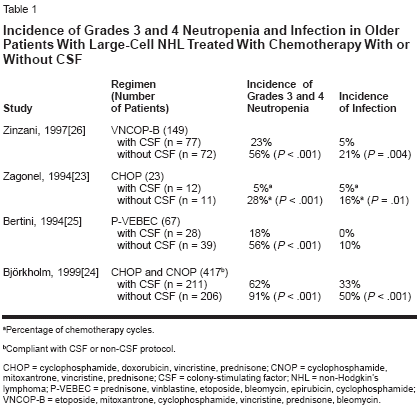
It has been shown that first-cyclemanagement with colony-stimulatingfactor (CSF) in both younger and olderpatients with non-Hodgkin's lymphomareduces the incidence and durationof neutropenia and its complicationsand helps maintain the chemotherapydose intensity. Four placebocontrolledrandomized trials of firstcycleCSF use with standard-doseCHOP and regimens with similarmyelosuppressive potential in elderlypatients with non-Hodgkin's lymphomahave reported significantlylower incidences of neutropenia andits complications in the CSF arms, by32% to 83% (Table 1).[23-26] In thetrial of CHOP and CHOP-like regimensby Zagonel and colleagues therates of grade 3 or 4 neutropenia andneutropenia-related infection were significantlylower in the patients whowere given CSF than in those who receivedplacebo; in addition, the use ofCSF was also associated with fewerand shorter treatment delays and withshorter lengths of stay in patients hospitalizedfor febrile neutropenia.[23]Jacobson and colleagues also reportedthat first-cycle CSF use made it possibleto deliver full-dose standard chemotherapyin elderly patients withnon-Hodgkin's lymphoma.[27]Risk Models in Non-Hodgkin'sLymphomaResearch is under way to determinerisk factors in order to develop a riskmodel for chemotherapy-induced neutropeniain patients with non-Hodgkin's lymphoma. This will makeit possible to target CSF to those athighest risk. Several risk factors havethus far been identified, mainly in retrospectivestudies. Since data indicatethat most complications of neutropeniaoccur early in the first few cyclesof chemotherapy, predictive risk studiesin non-Hodgkin's lymphoma haveassessed unconditional patient factorsfor the development of chemotherapyinducedneutropenia in the first cycle.A number of other risk factors havebeen identified, and predictive riskmodels that use these factors have beendeveloped. The risk factors seen repeatedlyin several studies[20, 28-31]include advanced age, poor performancestatus, planned high chemotherapydose intensity, the presenceof hepatic, renal, or cardiac comorbidities,and lack of primary prophylaxiswith CSF (Table 2). Less-obviouspretreatment factors-includinglow serum albumin levels, elevatedlactate dehydrogenase levels, bonemarrow involvement, and high levelsof soluble tumor necrosis factorreceptor-have also been identified,[20,28,31,32] but most of thesehave not yet been validated in separatepatient populations.Each risk factor for chemotherapyinducedneutropenia considered on itsown has limited predictive power, anda clinically useful risk model shouldconsider all of the risk factors in a patient.A pretreatment risk model developedthrough regression analysisfound five significant independent predictorsfor the first episode of febrileneutropenia.[33] These predictors ofearly febrile neutropenia were agegreater than 65 years, serum albuminlevel less than 35 g/L, planned doseintensity greater than 80% of the standard,absolute neutrophil count lessthan 1.5 109/L, and the presence ofhepatic disease.[33] A composite riskscore of the number of risk factorspresent differentiated patients as beingat low (1 or 2 factors) or high (≥ 3factors) risk for hospitalization for febrileneutropenia.A clinical decision model was thendeveloped to assess the cost implicationsof three strategies for using firstcycleCSF in patients treated withCHOP or CHOP-like regimens fornon-Hodgkin's lymphoma.[34] Thefirst treatment strategy was "control,"in which CSF was not used in any patients; the second was "universal," inwhich the CSF pegfilgrastim (Neulasta)was used in all patients in allcycles; and the third was "model," inwhich pegfilgrastim was used in allcycles in those patients who were athigh risk according to the model describedabove.[33] The "model" strategywas found to be the most cost-effective,because it targets pegfilgrastimprophylaxis to those who are mostlikely to benefit from it.Dose-Dense Chemotherapyin Non-Hodgkin's LymphomaMaintaining the dose intensity ofchemotherapy is associated withgreater overall survival in non-Hodgkin's lymphoma; therefore, it islogical to assume that increasing thedose intensity would further improvethe outcomes. Recent studies havetested this hypothesis, finding that thedose intensity can be increased byshortening the time between the chemotherapycycles, which also requiresprophylactic CSF in all cycles. A phaseII study in 120 patients with non-Hodgkin's lymphoma evaluated theefficacy of filgrastim in CHOP withCSF support given in 14-day cycles.This was compared with historical datafor CHOP given in the standard 21-day cycles.[35] In this study 90% ofthe cycles were given at full dose andon time and 86% of the patients weretreated with the planned six cycles.The preliminary results of a phaseIII trial of dose-dense (14-day) CHOPwith CSF support vs 21-day CHOP in738 patients older than 60 years havealso been reported.[36] At a medianfollow-up of 49 months, CHOP-14resulted in a significantly higher completeresponse rate (77.1% vs 63.5%,P = .028), greater overall survival(58.6% vs 44.6%, P = .002), andgreater disease-free survival (47% vs39.2%, P = .024) (Figure 2). TheCHOP-14 plus CSF regimen was alsowell tolerated, with a lower rate ofgrade 4 leukopenia than the CHOP-21 regimen (24% vs 44%).[37] Furthermore,appropriate supportive carewith CSF was required with dosedenseregimens; the rates of grades 3and 4 infections when filgrastim wasgiven for 10 days were half those whenit was given for only 7 days (11% vs21%).[38]The results from these studies arepromising, and suggest that the routineuse of dose-dense regimens withprophylactic CSF may prove to be animportant strategy for improving theoutcomes in patients with non-Hodgkin's lymphoma.ConclusionsThe incidence of and mortality innon-Hodgkin's lymphoma are increasing.This disease is potentially curable,but treatment failures are common andcan often be attributed, at least in part,to a failure to deliver adequate dosesof chemotherapy because of perceivedor actual problems with myelosuppression.Dose reductions and delaysare most common in elderly patients,and therefore, not unexpectedly, theresponse rates and overall survival arelower than those in younger patients.Several risk factors for chemotherapyinducedneutropenia and its complications(eg, hospitalization for febrileneutropenia and dose delays or reductions)have been identified in patientswith non-Hodgkin's lymphomatreated with standard-dose regimens.Incorporating these risk factors into apredictive model would make it possibleto determine those patients athighest risk for chemotherapy-inducedneutropenia and to appropriately targetfirst-cycle CSF to them.Furthermore, a recent study assessedthree strategies for prophylaxiswith CSF and found that the "model"strategy, in which the CSF pegfilgrastimis used only in those patientsat high risk on the basis of their compositerisk score, was the most costeffective.The promising results withdose-dense chemotherapy with CSFprophylaxis, providing greater overallsurvival in the elderly, should be confirmedin additional studies. Until thattime, a pretreatment predictive riskmodel for identifying patients at riskfor chemotherapy-induced neutropeniaand its complications should reducethe incidence and severity of chemotherapy-induced neutropenia bymaking the targeted use of CSF possiblein those most likely to benefitfrom it. Furthermore, it will facilitatethe cost-effective delivery of standardfull-dose chemotherapy on time.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.
Edwards BK, Howe HL, Ries LAG, et al:Annual report to the nation on the status ofcancer, 1973-1999, featuring implications ofage and aging on US cancer burden. Cancer94:2766-2792, 2002.
2.
Jemal A, Thomas A, Murran T, et al: Cancerstatistics, 2002. CA Cancer J Clin 52:23-47, 2002.
3.
Ries LA, Wingo PA, Miller DS, et al: Theannual report to the nation on the status of cancer,1973-1997, with a special section oncolorectal cancer. Cancer 88:2398-2424, 2000.
4.
American Cancer Society: Cancer Factsand Figures 2003. Atlanta, American CancerSociety, 2003.
5.
Glass AG, Karnell LH, Menck HR: TheNational Cancer Data Base report on non-Hodgkin’s lymphoma. Cancer 80:2311-2320,1997.
6.
Fisher RI, Gaynor ER, Dahlberg S, et al:Comparison of a standard regimen (CHOP)with three intensive chemotherapy regimens foradvanced non-Hodgkin’s lymphoma. N Engl JMed 328:1002-1006, 1993.
7.
Tirelli U, Errante D, van Glabbeke M, etal: CHOP is the standard regimen in patients ³70 years of age with intermediate-grade andhigh-grade non-Hodgkin’s lymphoma: Resultsof a randomized study of the European Organizationfor Research and Treatment of CancerLymphoma Cooperative Study Group. JClin Oncol 16:27-34, 1998.
8.
Coiffier B, Lepage E, Briere J, et al: CHOPchemotherapy plus rituximab compared withCHOP alone in elderly patients with diffuselarge-B-cell lymphoma. N Engl J Med 346:235-242, 2002.
9.
The International Non-Hodgkin’s LymphomaPrognostic Factors Project. A predictivemodel for aggressive non-Hodgkin’s lymphoma.N Engl J Med 329:987-994, 1993.
10.
Sonneveld P, de Ridder M, van der LelieH, et al: Comparison of doxorubicin andmitoxantrone in the treatment of elderly patientswith advanced diffuse non-Hodgkin’slymphoma using CHOP versus CNOP chemotherapy.J Clin Oncol 13:2530-2539, 1995.
11.
Balducci L, Extermann M: Managementof cancer in the older person: A practical approach.Oncologist 5:224-237, 2000.
12.
Zelenetz AD, Reider PA, Delgado DJ:Review of patterns of care among communityphysicians in intermediate grade NHL (IGL)reveals significantly greater planned and de-livered dose attenuation in older patients (abstract588). Blood 96:137a, 2000.
13.
Dixon DO, Neilan B, Jones SE, et al:Effect of age on therapeutic outcome in advanceddiffuse histiocytic lymphoma: TheSouthwest Oncology Group experience. J ClinOncol 4:295-305, 1986.
14.
Kwak LW, Halpern J, Olshen RA, et al:Prognostic significance of actual dose intensityin diffuse large-cell lymphoma: Results ofa tree-structured survival analysis. J Clin Oncol8:963-977, 1990.
15.
Epelbaum R, Faraggi D, Ben-Arie Y, etal: Survival of diffuse large cell lymphoma. Amultivariate analysis including dose intensityvariables. Cancer 66:1124-1129, 1990.
16.
Lepage E, Gisselbrecht C, Haioun C, etal: Prognostic significance of received relativedose intensity in non-Hodgkin’s lymphoma patients:Application to LNH-87 protocol. TheGELA (Groupe d’Etude des Lymphomes del’Adulte). Ann Oncol 4:651-656, 1993.
17.
Donnelly GB, Glassman J, Long C, etal: Granulocyte-colony stimulating factor (GCSF)may improve disease outcome in elderlypatients with diffuse large cell lymphoma(DLCL) treated with CHOP chemotherapy.Leuk Lymphoma 39:67-75, 2000.
18.
Kuderer N, Crawford J, Dale D, et al:Febrile neutropenia (FN) in adult lymphomapatients is associated with substantial mortalityand cost (abstract 3449). Blood 100:875a,2002.
19.
Bastion Y, Blay JY, Divine M, et al: Elderlypatients with aggressive non-Hodgkin’slymphoma: Disease presentation, response totreatment, and survival-A Groupe d’Etude desLymphomes de l’Adulte study on 453 patientsolder than 69 years. J Clin Oncol 15:2945-2953, 2001.
20.
Lyman GH, Morrison VA, Dale DC, etal: Risk of febrile neutropenia among patientswith intermediate grade non-Hodgkin’s lymphomareceiving CHOP chemotherapy. LeukLymphoma 44(12):2069-2076, 2003. See alsohttp://www.tandf.co.uk/journals.
21.
Caggiano V, Stolshek B, Delgado D, etal. First and all cycle febrile neutropenia hospitalizations(FNH) and costs in intermediategrade non-Hodgkins lymphoma (IGL) patientson standard-dose CHOP therapy (abstract1810). Blood 98:431a, 2000.
22.
Gomez H, Hidalgo M, Casanova L, etal: Risk factors for treatment-related death inelderly patients with aggressive non-Hodgkin’slymphoma: Results of a multivariate analysis.J Clin Oncol 16:2065-2069, 1998.
23.
Zagonel V, Babare R, Merola MC, et al:Cost-benefit of granulocyte colony-stimulatingfactor administration in older patients with non-Hodgkin’s lymphoma treated with combinationchemotherapy. Ann Oncol 5(suppl 2):127-132,1994.
24.
Björkholm M, Ãsby E, Hagberg H, etal: Randomized trial of r-metHu granulocytecolony-stimulating factor (G-CSF) as adjunctto CHOP or CNOP treatment of elderly patientswith aggressive non-Hodgkin’s lymphoma (abstract2655). Blood 94:599a, 1999.
25.
Bertini M, Freilone R, Vitolo U, et al: PVEBEC:A new 8-weekly schedule with orwithout rG-CSF for elderly patients with aggressivenon-Hodgkin’s lymphoma (NHL). AnnOncol 5:895-900, 1994.
26.
Zinzani PL, Pavone E, Storti S, et al:Randomized trial with or without granulocytecolony-stimulating factor as adjunct to inductionVNCOP-B treatment of elderly high-gradenon-Hodgkin’s lymphoma. Blood 89:3974-3979, 1997.
27.
Jacobson JO, Grossbard M, ShulmanLN, et al: CHOP chemotherapy with preemptivegranulocyte colony-stimulating factor inelderly patients with aggressive non-Hodgkin’slymphoma: A dose-intensity analysis. ClinLymphoma 1:211-218, 2000.
28.
Kloess M, Wunderlich A, Truemper L,et al: Predicting hematotoxicity in multicyclechemotherapy (abstract 381). Blood 94:87a,1999.
29.
Morrison VA, Picozzi V, Scott S, et al:The impact of age on delivered dose intensityand hospitalizations for febrile neutropenia inpatients with intermediate-grade non-Hodgkin’s lymphoma receiving initial CHOPchemotherapy: A risk factor analysis. Clin Lymphoma2:47-56, 2001 .
30.
Chrischilles E, Delgado DJ, Stolshek BS,et al: Impact of age and colony-stimulating factoruse on hospital length of stay for febrileneutropenia in CHOP-treated non-Hodgkin’slymphoma. Cancer Control 9:203-211, 2002.
31.
Voog E, Bienvenu J, Warzocha K, et al:Factors that predict chemotherapy-inducedmyelosuppression in lymphoma patients: Roleof the tumor necrosis factor ligand-receptor system.J Clin Oncol 18:325-331, 2000.
32.
Intragumtornchai T, Sutheesophon J,Sutcharitchan P, et al: A predictive model forlife-threatening neutropenia and febrile neutropeniaafter the first course of CHOP chemotherapyin patients with aggressive non-Hodgkin’s lymphoma. Leuk Lymphoma37:351-360, 2000.
33.
Lyman GH, Delgado D: Risk and timingof hospitalization for febrile neutropeniaamong patients receiving CHOP and CHOPlikeregimens for intermediate grade non-Hodgkin’s lymphoma (abstract 3085). Blood100:780a, 2002.
34.
Lyman GH, Kuderer NM, Crawford J,et al: Economic impact of pegfilgrastim usebased on the risk of febrile neutropenia (FN)in NHL patients treated with CHOP (abstract2384). Proc Am Soc Clin Oncol 22:593, 2003.
35.
Gregory SA, Case DC Jr, Bosserman L,et al: Fourteen-day CHOP supported withfilgrastim in patients with aggressive non-Hodgkin’s lymphoma: Results of a phase IIstudy. Clin Lymphoma. In press.
36.
Pfreundschuh M, Trümper L, Kloess M,et al: 2-Weekly CHOP (CHOP-14): The newstandard regimen for patients with aggressivenon-Hodgkin’s lymphoma (NHL) >60 years ofage (abstract 3027). Blood 98:725a, 2001.
37.
Wunderlich A, Kloess M, Reiser M, etal: Practicability and acute haematological toxicityof 2- and 3-weekly CHOP and CHOEPchemotherapy for aggressive non-Hodgkin’slymphoma: results from the NHL-B trial of theGerman High-Grade Non-Hodgkin’s LymphomaStudy Group (DSHNHL). Ann Oncol14:881-893, 2003.
38.
Kloess M, Zeynalova S, Truemper L, etal: Effects of G-CSF schedule on leukocyterecovery and infection rate in the CHOP-14regimen for elderly patients with aggressivelymphoma (abstract 2401). Proc Am Soc ClinOncol 22:597, 2003.
Oncology Peer Review On-The-Go: Cancer-Related Fatigue Outcome Measures in Integrative Oncology
September 20th 2022Authors Dori Beeler, PhD; Shelley Wang, MD, MPH; and Viraj A. Master, MD, PhD, spoke with CancerNetwork® about a review article on cancer-related fatigue published in the journal ONCOLOGY®.