The Role of Primary Tumor Treatment and Metastasis-Directed Therapy in Oligometastatic Prostate Cancer
This case looks at an otherwise healthy 59-year-old man who was recently diagnosed with clinical stage IVB prostate adenocarcinoma. What is the most appropriate management for this patient?
Oncology (Williston Park). 33(5):187-91.

Abhinav Gupta

Thomas J. Pugh, MD

Elaine Lam, MD

Alison D. Sheridan, MD

Sameer Nath, MD

Figure 1. Images Showing a PIRADS 5 Lesion in the Right Lateral Peripheral Zone in the Mid Gland
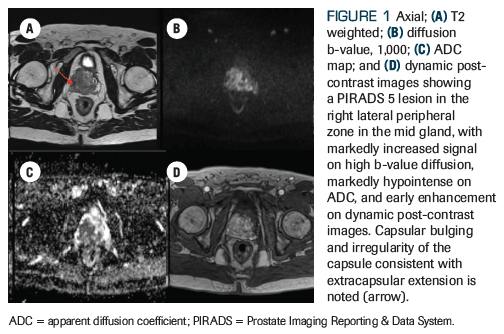
Figure 2. Coronal T2-Weighted Images Showing a PIRADS 5 Lesion
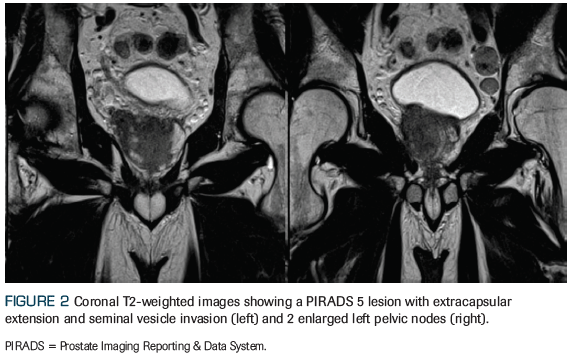
Figure 3. Whole-Body Bone Scintigraphy Showing Increased Focal Radiotracer Uptake
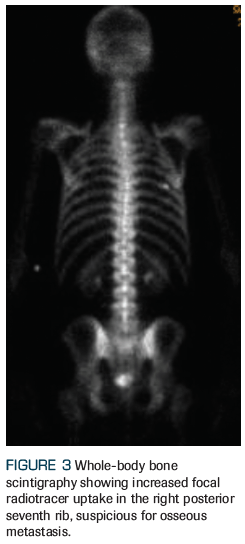
Figure 4. Radiation Treatment to the Prostate
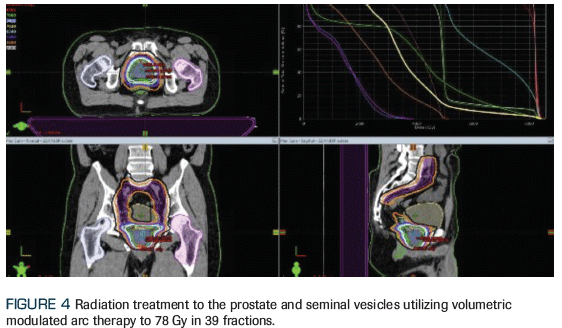
Figure 5. Radiation Treatment to the Right Seventh Rib Osseous Metastasis
The Case
An otherwise healthy 59-year-old man who was recently diagnosed with American Joint Committee on Cancer (AJCC) 8th edition clinical stage IVB (T3aN1M1b) prostate adenocarcinoma presents to the radiation oncology clinic for consideration of radiation therapy. After he was found to have an elevated prostate-specific antigen (PSA) level of 16.7 ng/mL, he underwent a standard transrectal ultrasound–guided biopsy that revealed a prognostic grade group 4 (Gleason score 4+4) in 7 of 12 core biopsies, with lower-grade adenocarcinoma present in the remaining 5 core biopsies. A multiparametric prostate MRI revealed a large (4 cm) Prostate Imaging Reporting and Data System (PIRADS) 5 lesion with extracapsular extension, as well as pelvic lymphadenopathy, including a 2.2-cm left internal iliac node (Figures 1 and 2). A staging bone scan showed focal uptake in the seventh right rib (Figure 3), corresponding to a sclerotic lesion on follow-up CT, which was deemed highly suspicious for an isolated focus of metastatic prostate cancer after careful review in multidisciplinary conference. He was started on upfront systemic therapy with leuprolide, abiraterone acetate, and prednisone. Three months later, his PSA level had decreased to 0.03 ng/mL, and 1 month after that, imaging showed a mixed response to systemic therapy with reduced bilateral pelvic lymphadenopathy and persistent focal uptake in the seventh right rib metastatic lesion. No new lesions were identified.
What is the most appropriate management for this patient?
A. Radical prostatectomy with pelvic lymph node dissection
B. Pelvic external beam radiotherapy (EBRT) and stereotactic body radiation therapy (SBRT) to the bony metastatic lesion
C. Continue with current systemic therapy
D. Intensify systemic therapy with6 cycles of docetaxel
CORRECT ANSWER:B.Pelvic external beam radiotherapy (EBRT) and stereotactic body radiation therapy (SBRT) to the bony metastatic lesion
Discussion
Cancer is thought to progress in an orderly stepwise fashion, from a primary organ, to regional metastatic sites, and then to distant metastatic sites. In the past, cancer was traditionally viewed as a systemic disease when it spread to distant organs, and local therapy was deemed unlikely to improve outcomes or render cure. However, pioneering work from Hellman and Weichselbaum in 1995 led to the hypothesis that cancers may instead fall along a continuum between localized and systemic disease. They proposed that a transitional stage between local and metastatic cancer exists, known as “oligometastatic cancer.”[1] In this intermediate state, metastases are initially more limited before becoming widespread, and local therapies could still provide a benefit, perhaps even cure. Many experts have explored this hypothesis in a variety of solid tumor sites.[2-4] The exact definition of oligometastatic disease remains controversial, but studies have typically included patients with one to five metastatic sites identified on standard imaging.[5,6] With the advent of novel molecular imaging, including prostate-specific membrane antigen (PMSA) PET, carbon-11/fluorine (F)-18 fludeoxyglucose PET, and F-18 fluciclovine PET,[7,8] we can now detect disease spread at lower PSA levels than ever before. As a result, the prevalence of oligometastatic prostate cancer has increased, as have discussions of whether or not these patients should be treated differently than those with overtly widespread metastatic disease.
Metastatic prostate cancer has traditionally been treated with androgen deprivation therapy (ADT), with an initial goal of lowering serum testosterone to castrate levels.[9] The optimal form of ADT has not been determined with level 1 evidence, and options include medical castration with gonadotropin-releasing hormone agonist (GnRH) agonists/antagonists or surgical orchiectomy. Treatment-related side effects of ADT can adversely impact quality of life, and include fatigue, mood changes, hot flashes, decreased libido, bone fracture, cardiovascular disease, and metabolic changes.[10]
Recent clinical trials have shown the benefit of adding other systemic therapies to standard ADT in patients with metastatic prostate cancer. In particular, the addition of abiraterone acetate or docetaxel to standard ADT has been shown to improve overall survival in select high-risk men.[11,12] There are limited data comparing these two regimens, and both appear to be associated with the same magnitude of survival benefit.[13] Interestingly, the extent of survival improvement has been shown to differ between low- and high-burden metastatic disease. For example, adding docetaxel to ADT fails to improve survival outcomes in low-burden disease or oligometastatic disease, while demonstrating improved outcomes in those with high-burden disease.[14] This also supports the notion that low-burden disease metastases may behave differently than a more widespread disease process.
Treatment of the primary tumor in metastatic prostate cancer was always considered a palliative measure for patients suffering from medically refractory local symptoms. However, this changed when a benefit from local therapy was observed in other types of metastatic cancers, sparking an interest in local therapy as a potentially life-prolonging treatment for metastatic prostate cancer. Cytoreductive therapy for stage IV malignancies has been associated with improved outcomes in several solid cancer types, including colon,[15] ovarian,[16] and renal cell carcinoma.[17]
The underlying theory is that cytoreduction can help eliminate the original source of tumor spread and highest concentration of tumor clonogens, which may improve disease control when combined with effective systemic therapy to treat the remaining microscopic distant disease. Recently, a growing body of retrospective studies has indicated a potential benefit of local therapy in metastatic prostate cancer.[18-22] In an analysis of the Surveillance, Epidemiology, and End Results (SEER) database, Culp et al identified 8,185 men with documented stage IV (M1) disease, of whom 245 underwent definitive prostatectomy and 129 underwent brachytherapy. Patients undergoing local therapy had improved survival compared with those without any local therapy. Factors associated with increased cancer-specific mortality in patients undergoing local therapy included T4 disease, high grade, and PSA level > 20 ng/mL.[18] In a follow-up SEER analysis, the benefit of local therapy was highest in patients with a cancer-specific mortality risk of < 40%.[20]
Two recent analyses of the National Cancer Database have also found that local therapy was associated with improved overall survival compared with ADT alone. Rusthoven et al identified 6,382 men in this database who were diagnosed with metastatic prostate cancer between 2004 and 2012 and were treated with ADT with or without radiation.[23] Only a small number of men (8.4%) received prostate-directed radiation. After a median follow-up of 5.1 years, the 5-year overall survival rate was 49% with the addition of radiation compared with 33% with ADT alone after adjusting for covariates with propensity score matching. Secondary analysis showed that men treated with ADT plus prostatectomy also had superior survival compared with men treated with ADT alone; there was no difference between the radiation or prostatectomy cohorts in terms of survival. These results were validated in a separate analysis of the National Cancer Database.[24]
Finally, a variety of additional retrospective and post-hoc studies have also indicated a benefit of local therapy in stage IV prostate cancer patients. In particular, in node-positive (stage IV) disease, the addition of radical prostatectomy to conventional treatment has been associated with improved clinical outcomes in several studies.[25-28] A post-hoc analysis of the Southwest Oncology Group 8894 trial showed that patients with metastatic prostate cancer who underwent radical prostatectomy prior to systemic treatment had improved cancer-specific survival.[29] Similarly, using a case control design study, Heidenreich et al found increased time to castration resistance, freedom from clinical progression, and improved overall survival in men who received a radical prostatectomy in the setting of low-volume skeletal metastases.[30]
Although these studies provide promising data for the potential role of prostate-directed therapy in the setting of metastatic prostate cancer, they were retrospective in nature and are therefore limited by the possibility/likelihood of selection bias. There are two recently published randomized trials evaluating the impact of prostate radiation therapy in M1 patients. The HORRAD trial[31] is a multicenter randomized controlled trial conducted in the Netherlands, which began in 2004. Men with newly diagnosed prostate cancer, bone metastases, and a PSA level > 20 ng/mL were randomized to ADT alone or ADT plus radiotherapy to the prostate. The primary endpoint was overall survival; the investigators ambitiously estimated a 10-month absolute improvement in overall survival with the addition of prostate EBRT to ADT. After a median follow-up of 47 months, prostate EBRT significantly improved PSA failure–free survival (hazard ratio [HR], 0.78; 95% CI, 0.63–0.97; P = .02). However, prostate EBRT was not associated with a statistically significant improvement in median overall survival (43 months with ADT alone vs 45 months with prostate EBRT and ADT). There are several important considerations when applying HORRAD to current medical practice. First, the relatively large estimated survival impact from prostate EBRT likely underpowered the trial to detect smaller, yet clinically significant, differences in overall survival between the two groups. Secondly, the median PSA level for the entire cohort was 142 ng/mL, and there was no limit to the number of allowable metastatic lesions detected on bone scan. Furthermore, a staging CT scan was not required, meaning the presence of nodal or visceral metastases was unknown. Therefore, patients enrolled in HORRAD had a disproportionately high metastatic burden and may not be representative of the oligometastatic patient population. Interestingly, on subgroup multivariate analysis, there was a trend towards an overall survival benefit for patients with < 5 bone lesions (HR, 0.68; 95% CI, 0.42–1.10). Considering the inherent limitations of unplanned subset analyses, these data should be considered hypothesis generating only.
The second published randomized trial was an analysis of STAMPEDE participants.[32] These investigators actually prospectively tested whether or not prostate radiotherapy improved overall survival over systemic therapy alone in M1 patients and specifically in those men with low metastatic disease burden (as defined in the CHAARTED trial). In the M1 cohort at large, STAMPEDE showed similar results to HORRAD, in which progression-free survival was improved with the addition of prostate radiation therapy, without a statistically significant improvement in overall survival. However, in those men with low metastatic disease burden, prostate EBRT significantly improved overall survival compared with systemic therapy alone (HR, 0.68; 95% CI, 0.52–0.90).
We now have level 1 evidence demonstrating improved progression-free survival with the addition of prostate EBRT to ADT in M1 patients and, more specifically, an overall survival advantage in M1 patients with low metastatic disease burden as determined by appropriately powered a priori hypothesis testing. Furthermore, these benefits are highly consistent in both outcome and magnitude with the preceding hypothesis-generating data.
A growing number of patients may present with either synchronous or metachronous oligometastatic disease, and the optimal management of these patients is unclear. SBRT is a highly conformal mode of radiation therapy that has been associated with high rates of local control. It is a particularly attractive option due to its favorable toxicity profile and short treatment duration. Two recent clinical trials provide supportive data for metastasis-directed therapy for patients who present with limited metastatic disease.
The STOMP trial was a multicenter phase II trial that randomized patients with biochemical recurrence after systemic therapy, three or fewer extracranial metastatic lesions on PSMA PET imaging, and serum testosterone levels greater than 50 ng/mL to either metastasis-directed therapy (including SBRT or surgical extirpation) to all active lesions or surveillance. PSA surveillance was conducted every 3 months, and biochemical progression prompted repeat imaging. Patients who developed symptomatic disease, locoregional tumor progression, or more than three extracranial metastatic lesions were started on ADT. The primary endpoint was ADT-free survival. A total of 62 patients were enrolled. After a median follow-up of 3 years, the median ADT-free survival was 13 months in the surveillance group vs 21 months in the SBRT group. Treatment was well tolerated, and there were no grade 2 to 5 toxicities.[33]
A similar clinical trial, POPSTAR, recently corroborated these findings. In this prospective trial, 33 patients with 1 to 3 oligometastases were treated with a single fraction of SBRT to each lesion. In all, SBRT was feasible for 97% of putative targets. After a median of 2 years’ follow-up, the local progression–free survival rate was 93% and the distant progression–free survival rate was 39%. Of the 22 men not on any form of ADT, the 2-year rate of freedom from ADT was 48%. There was one grade 3 complication (vertebral body collapse). No differences in quality of life were found.[34] Overall, these small prospective studies provide compelling evidence for the use of metastasis-directed therapy as a safe and effective option for those with low-volume metastatic disease to decrease the risk for disease progression and delay the use of ADT.
Outcome of This Case
After a discussion of the pros and cons of various management options, the patient elected to proceed with fractionated radiotherapy to the pelvis and SBRT to the bone lesion (Figures 4 and 5), along with continuous GnRH agonist therapy with abiraterone acetate plus prednisone. The patient tolerated radiotherapy well, with no significant toxicity. At this time, he is still receiving adjuvant ADT, with a PSA level < 0.01 ng/mL. We plan to discontinue ADT and allow testosterone recovery after completion of 24 months of continuous therapy.
Conclusion
In summary, oligometastatic prostate cancer is being increasingly viewed as a unique entity separate from widely spread metastatic disease. A growing body of evidence now supports a role for local ablative therapy to the primary tumor and metastatic sites to improve disease control and potentially delay the use of ADT and its associated side effects. Novel imaging modalities will likely increase the detection of limited metastatic sites, demonstrating the importance of understanding the benefits of local or ablative therapy in this clinical scenario. Overall, radiation therapy offers a noninvasive approach that can be used to treat both the primary tumor site and metastatic sites, with a favorable toxicity profile. At present, select patients with a good performance status, limited disease sites amenable to SBRT, and a strong desire to avoid the side effects of long-term ADT may be considered for consolidative therapy after a discussion of goals of care, treatment alternatives, and shared decision making. In the future, more granular risk stratification with next-generation imaging and the integration of genomic results may help determine exactly which patients with metastatic prostate cancer will benefit most from local therapy.
KEY POINTS
• Oligometastatic prostate cancer may be a biologically unique entity.
• Select patients with limited sites of metastatic disease benefit from prostate-directed and metastasis-directed therapy.
• Two randomized controlled trials support a progression-free survival benefit with the addition of prostate radiation therapy to systemic therapy; this benefit has translated into an overall survival advantage in select patients with low-burden metastatic disease.
• Future trials are needed to better elucidate the risks and benefits of other local therapies, such as surgery. Understanding the role of local therapy in the setting of multiple systemic therapy options will also require additional study.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8-10.
2. Shultz DB, Filippi AR, Thariat J, et al. Stereotactic ablative radiotherapy for pulmonary oligometastases and oligometastatic lung cancer. J Thorac Oncol. 2014;9:1426-33.
3. Salama JK, Chmura SJ. The role of surgery and ablative radiotherapy in oligometastatic breast cancer. Semin Oncol. 2014;41:790-7.
4. Weiser MR, Jarnagin WR, Saltz LB. Colorectal cancer patients with oligometastatic liver disease: what is the optimal approach? Oncology (Williston Park). 2013;27:1074-8.
5. Tosoian JJ, Gorin MA, Ross AE, et al. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol. 2017;14:15-25.
6. Singh D, Yi WS, Brasacchio RA, et al. Is there a favorable subset of patients with prostate cancer who develop oligometastasis? Int J Radiat Oncol Biol Phys. 2004;58:3-10.
7. Evangelista L, Briganti A, Fanti S, et al. New clinical indications for (18)F/(11)C-choline, new tracers for positron emission tomography and a promising hybrid device for prostate cancer staging: a systematic review of the literature. Eur Urol. 2016;70:161-75.
8. Evans JD, Jethwa KR, Ost P, et al. Prostate cancer-specific PET radiotracers: a review on the clinical utility in recurrent disease. Pract Radiat Oncol. 2018;8:28-39.
9. Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467-79.
10. Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388-99.
11. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163-77.
12. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352-60.
13. Wallis CJD, Klaassen Z, Bhindi B, et al. Comparison of abiraterone acetate and docetaxel with androgen deprivation therapy in high-risk and metastatic hormone-naive prostate cancer: a systematic review and network meta-analysis. Eur Urol. 2018;73:834-44.
14. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737-46.
15. Temple LK, Hsieh L, Wong WD, et al. Use of surgery among elderly patients with stage IV colorectal cancer. J Clin Oncol. 2004;22:3475-84.
16. Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248-59.
17. Michkisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966-70.
18. Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014;65:1058-66.
19. Antwi S, Everson TM. Prognostic impact of definitive local therapy of the primary tumor in men with metastatic prostate cancer at diagnosis: a population-based, propensity score analysis. Cancer Epidemiol. 2014;38:435-41.
20. Fossati N, Trinh QD, Sammon J, et al. Identifying optimal candidates for local treatment of the primary tumor among patients diagnosed with metastatic prostate cancer: a SEER-based study. Eur Urol. 2015;67:3-6.
21. Leyh-Bannurah SR, Gazdovich S, Budäus L, et al. Local therapy improves survival in metastatic prostate cancer. Eur Urol. 2017;72:118-24.
22. Satkunasivam R, Kim AE, Desai M, et al. Radical prostatectomy or external beam radiation therapy vs no local therapy for survival benefit in metastatic prostate cancer: a SEER-Medicare analysis. J Urol. 2015;194:378-85.
23. Rusthoven CG, Jones BL, Flaig TW, et al. Improved survival with prostate radiation in addition to androgen deprivation therapy for men with newly diagnosed metastatic prostate cancer. J Clin Oncol. 2016;34:2835-42.
24. Löppenberg B, Dalela D, Karabon P, et al. The impact of local treatment on overall survival in patients with metastatic prostate cancer on diagnosis: a National Cancer Data Base analysis. Eur Urol. 2017;72:14-9.
25. Ghavamian R, Bergstralh EJ, Blute M, et al. Radical retropubic prostatectomy plus orchiectomy versus orchiectomy alone for pTxN+ prostate cancer: a matched comparison. J Urol. 1999;161:1223-7.
26. Steuber T, Budäus L, Walz J, et al. Radical prostatectomy improves progression-free and cancer-specific survival in men with lymph node positive prostate cancer in the prostate-specific antigen era: a confirmatory study. BJU Int. 2011;107:1755-61.
27. Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472-9.
28. Schröder FH, Kurth KH, Fossa SD, et al. Early versus delayed endocrine treatment of pN1-3 M0 prostate cancer without local treatment of the primary tumor: results of European Organisation for the Research and Treatment of Cancer 30846: a phase III study. J Urol. 2004;172:923-7.
29. Tangen CM, Hussain MH, Higano CS, et al. Improved overall survival trends of men with newly diagnosed M1 prostate cancer: a SWOG phase III trial experience (S8494, S8894 and S9346). J Urol. 2012;188:1164-9.
30. Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J Urol. 2015;193:832-8.
31. Boeve L, Hulshof M, Vis A, et al. Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomized clinical trial: data from the HORRAD trial. Eur Urol. 2019;75:410-8.
32. Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomized controlled phase 3 trial. Lancet. 2018;392:2353-66.
33. Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446-53.
34. Siva S, Bressel M, Murphy DG, et al. Stereotactic ablative body radiotherapy (SABR) for oligometastatic prostate cancer: a prospective clinical trial. Eur Urol. 2018;74:455-62.
