When Surgery Is Not an Option in Renal Cell Carcinoma: The Evolving Role of Stereotactic Body Radiation Therapy
This review summarizes the current evidence supporting the use of SBRT as treatment for inoperable renal cell carcinoma, as well as provides recommendations for patient selection and reviews the technical aspects of treatment and the expected toxicities.
Oncology (Williston Park). 33(5):167-73, 177.

Joseph Miccio, MD

Kimberly Johung, MD, PhD

Table 1. Published Prospective Studies of SBRT in the Treatment of Localized RCC
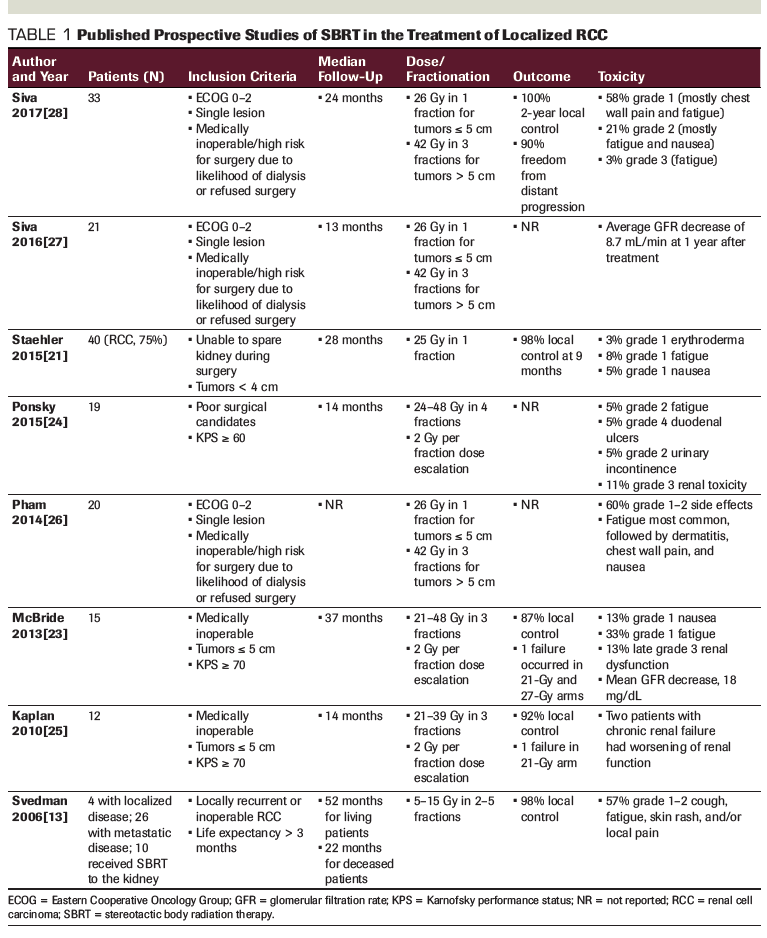
Table 2. Published Retrospective Studies of SBRT in the Treatment of Localized RCC
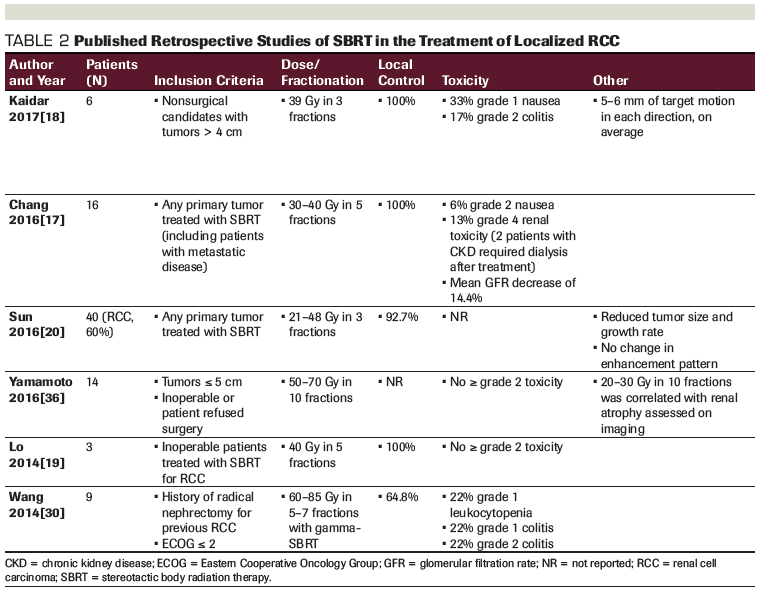
Table 3. Technical Approaches of Published Prospective Studies of SBRT in Patients With RCC
Historically, radiation therapy has played a limited role in the management of renal cell carcinoma because early studies showed that it had no benefit in the neoadjuvant or adjuvant settings. Thus, radiation has typically been employed for only palliation of metastatic sites. As the ability to deliver conformal high-dose-per-fraction radiation became available, studies began to show excellent local control when treating oligometastatic sites of renal cell carcinoma with stereotactic body radiation therapy (SBRT). Recently, SBRT has been studied in the management of the primary tumor in nonsurgical patients with localized renal cell carcinoma. Excellent local control rates and low rates of treatment-related toxicity were reported with single-fraction (26 Gy) and multi-fraction (36 to 45 Gy in 3 fractions or 40 to 50 Gy in 5 fractions) regimens. While the evidence to date is limited by small cohort sizes and variability in treatment approaches, the reported outcomes are promising. Ongoing studies will continue to define how renal SBRT fits into the management of patients who are not eligible for surgery.
Introduction
Curative-intent treatment of localized renal cell carcinoma entails surgical resection with a radical or partial nephrectomy. Early randomized studies failed to show a survival benefit with the addition of neoadjuvant[1,2] or adjuvant[3,4] radiotherapy. Moreover, renal cell carcinoma is considered a radioresistant histology based on in vitro studies.[5] Thus, radiation therapy has historically been limited to the palliative setting for patients with renal cell carcinoma. Stereotactic radiosurgery (SRS) has long been used for effective local control of intracranial metastases from renal cell carcinoma, most often with a single fraction of high-dose radiation therapy.[6-9] With the advent of technology allowing for safe delivery of highly conformal high-dose-per-fraction radiation therapy to extracranial sites, stereotactic body radiation therapy (SBRT) is now widely used to treat oligometastatic disease. Excellent local control rates of higher than 90% at 1 year have been demonstrated for metastatic renal cell carcinoma lesions in the spine[8,10-12] and visceral sites.[9,13-15] The findings of these clinical studies coincided with another study demonstrating the efficacy of ablative high-dose-per-fraction radiation therapy in treating renal cell carcinoma in a mouse model.[16] These results suggest that ablative doses of radiation therapy delivered with stereotactic techniques have the potential to impact outcomes in renal cell carcinoma, whereas the role of standard fractionated radiation therapy is limited.
In light of the promising outcomes seen with SBRT for metastatic sites of renal cell carcinoma, renal SBRT is increasingly being evaluated for treatment of the primary tumor in nonsurgical candidates. There are several retrospective[17-20] and prospective[13,21-28] studies evaluating the role of SBRT in the treatment of non-metastatic renal cell carcinoma, which have demonstrated excellent local control rates with minimal toxicity, and additional studies are ongoing. This review summarizes the current evidence supporting the use of SBRT as treatment for inoperable renal cell carcinoma, with a focus on prospective studies. We will provide recommendations for patient selection and review the technical aspects of treatment, as well as the expected toxicities.
Review of the Evidence for SBRT in Localized Renal Cell Carcinoma
Prospective studies
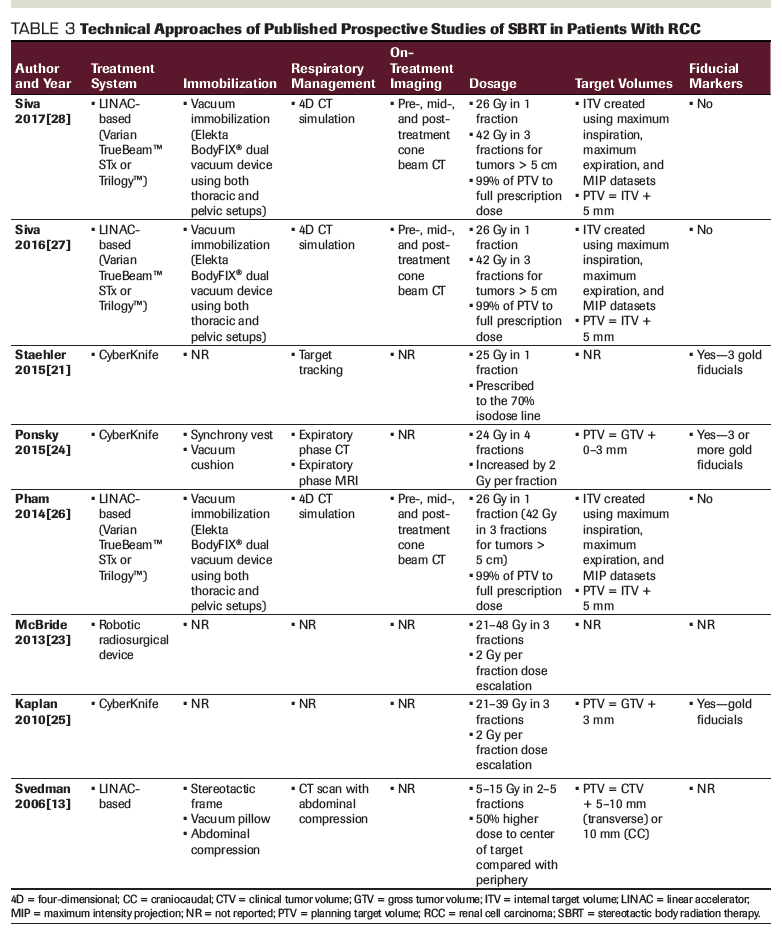
Although only a limited number of prospective studies evaluating SBRT in localized renal cell carcinoma have been published, the results are encouraging (Table 1). Interpretation of these studies is limited by the small number of patients treated, lack of long-term follow-up, and the varying dose fractionation schemes. The trials evaluated cohorts of 10 to 40 medically inoperable patients treated with doses of 25 to 26 Gy in 1 fraction, 24 to 48 Gy in 4 fractions, or 21 to 48 Gy in 3 fractions. With an average follow-up of 13 to 52 months, local control rates ranged from 87% to 100%. In the most recently published prospective series, 33 patients with renal cell carcinoma received SBRT to the primary tumor (26 Gy in 1 fraction or 42 Gy in 3 fractions for larger tumors), and the 2-year local control rate was 100%.[28] A larger study published in 2015 included 40 patients who received 25 Gy in a single fraction to primary renal tumors (75% of which were renal cell carcinoma), and the 9-month local control rate was 98%, though longer follow-up is lacking.[21] While these prospective studies demonstrate promising rates of local control with SBRT, there are no completed randomized phase III trials to date comparing SBRT with other local therapy modalities.
The prospective dose escalation studies evaluated doses ranging from 21 to 48 Gy in 3 fractions[23,25] and 24 to 48 Gy in 4 fractions.[24] For the 3-fraction studies, local failure only occurred in 1 patient receiving 21 Gy and 1 patient receiving 27 Gy.[23,25] These results suggest that for 3-fraction regimens, a minimum of 11 Gy per fraction should be considered, which is the minimum dose at which no patient in these studies experienced a local failure. This is consistent with the consensus statement from the International Radiosurgery Oncology Consortium for Kidney, which recommends a total dose of 36 to 45 Gy for 3-fraction regimens.[29] For 5-fraction regimens, a total dose of 40 to 50 Gy is recommended. In the 4-fraction dose-escalation study, the local control rate for the 15 patients with evaluable responses was 100%.[24] The study did not report on toxicity levels for individual doses, but overall, no dose-limiting toxicity was reached (defined as any treatment-related ≥ grade 3 gastrointestinal/genitourinary toxicity or any grade 4 toxicity). For treatment in a single fraction, 25 to 26 Gy has been studied,[21,22,26,28] with no significant difference in toxicities or negative impact on local control rates compared with multi-fraction regimens.[24-28] Thus, multiple fractionation schemes appear to be effective. While studies are lacking to compare these regimens, total dose and fraction size should be influenced by the size of the primary tumor and its proximity to the adjacent normal tissues.
Retrospective studies
Retrospective studies of SBRT in place of surgery for renal cell carcinoma also showed excellent local control outcomes, ranging from 64.8% to 100% (Table 2). The largest series of 40 patients with renal tumors (60% of which were renal cell carcinoma) reported a 92.7% local control rate with 21 to 48 Gy in 3 fractions, with a median follow-up of approximately 18 months.[20] In the second largest series of 16 patients treated with 30 to 40 Gy in 5 fractions, the local control rate was 100%, with a median follow-up of 19 months.[17] Only one study demonstrated a suboptimal 1-year local control rate, of 64.8%, for patients treated with 60 to 85 Gy in 5 to 7 fractions, but the study population included patients with more advanced disease who had failed in the contralateral kidney after a prior nephrectomy.[30] The efficacy of renal SBRT is further supported by a meta-analysis of both prospective and retrospective studies, which showed an estimated weighted 2-year local control rate of 92.9%.[31]
Technical considerations
Delivery of precise, highly conformal ablative doses of radiation to the kidney requires reproducible patient setup and appropriate respiratory motion management. Technical approaches utilized in published studies are varied (Table 3). Respiratory motion management is essential to deliver a highly conformal treatment because the mean displacement of the kidneys with respiration is 0.75 cm, with a range of 0.10 to 2.15 cm for the left kidney and 0.11 to 1.92 for the right kidney.[32] Most displacement occurs in the superior-inferior direction. Furthermore, on-treatment imaging is essential given variability in patient setup.[33] With dual-vacuum immobilization (or Vac-LokTM™ Cushions with abdominal compression), the average three-dimensional displacement of the target post-treatment was restricted to 1 mm, with a maximal displacement in any direction of ≤ 3 mm. This suggests that, with adequate immobilization, respiratory excursion and interfraction motion is limited, and respiratory gating methods may not be essential.[34] The most recent prospective series used LINAC-based SBRT without fiducials and a four-dimensional (4D) CT simulation for respiratory motion management.[22,28] Other series utilizing CyberKnife® treatment platforms employed real-time target tracking of implanted gold fiducials.[21,24] Planning tumor volume (PTV) expansions differ based on treatment platform, with real-time target tracking of gold fiducials and CyberKnife® utilizing a PTV of 0.0 to 3.0 mm,[24] and LINAC-based SBRT based on 4D CT with no fiducials or target tracking utilizing a PTV of 5.0 mm.[22,28] Tables detailing suggested dose constraint values for different dose/fractionation schemes are reported by Pham[26] and Siva.[29]
Response evaluation
One of the challenges of SBRT is the interpretation of post-treatment radiographic studies, and defining characteristics that coincide with treatment response or recurrence. Renal cell carcinoma is generally slow-growing, and it has a similarly slow radiographic response to SBRT. The largest study of tumor response included 41 tumors treated with SBRT. The mean pretreatment linear growth rate was 0.68 cm per year; post-treatment, the linear growth rate regressed by an average of 0.37 cm per year. The study found no significant changes in enhancement on CT or MRI when comparing pre- and post-treatment imaging.[20]
Toxicities
In general, toxicity rates for SBRT in primary renal cell carcinoma are favorable (Tables 1 and 2). In the largest prospective series evaluating SBRT to 25 Gy in 1 fraction for renal tumors, there were no grade > 1 toxicities.[21] In the most recent prospective study, only 1 patient developed grade 3 toxicity (fatigue), with 21% experiencing grade 2 toxicity (mostly fatigue and nausea), and 58% of patients experiencing grade 1 toxicity, mostly comprised of chest wall pain and fatigue.[28]
Of concern is the potential for SBRT to impact renal function. A study of 21 patients treated with SBRT showed that the glomerular filtration rate (GFR) decreased by 8.7 mL per minute on average at 1 year post-treatment.[22] Similarly, in a pooled analysis of 223 patients treated with renal SBRT, the average GFR decreased by ~5.5 mL per minute after SBRT.[35] For more fractionated regimens, a dose distribution of 20 to 30 Gy in 10 fractions had a strong correlation with renal atrophy as assessed on CT scan.[36] In a case report of a patient treated with 26 Gy in 1 fraction, regional kidney function after SBRT was assessed with 99mTc dimercaptosuccinic acid single photon emission tomography/CT, and renal function was preserved in regions receiving less than 13 Gy in a single fraction.[37] Although most nephrotoxicity experienced with renal SBRT is subclinical, toxicity is increased in patients with underlying chronic kidney disease (CKD), with reported cases of stage 4 to 5 CKD requiring dialysis after SBRT.[17] The low toxicity rates may be due to the parallel function of nephrons, allowing for preserved renal function if a substantial portion of normal kidney is spared. Long-term toxicity after SBRT, however, is unknown.
Patient selection
Surgery remains the standard curative-intent therapy for localized renal cell carcinoma. Thus, SBRT for renal cell carcinoma should be considered only in patients who have contraindications to surgery. Some studies also treated renal cell carcinoma patients with SBRT if they refused surgery.[22,26,28] However, patients should be educated that surgery is the standard of care prior to considering SBRT in this population. Recent German guidelines on the role of radiation therapy in renal cell carcinoma state that SBRT is currently considered an experimental option due to the small number of patients reported in the literature, though it will likely have an increased role in the future as results from ongoing studies continue to emerge.[38]
Renal SBRT has also been studied in several other patient populations, including patients with metastatic disease as an alternative to cytoreductive nephrectomy,[39] those with bilateral renal tumors,[30,40] and those with a contralateral recurrence after nephrectomy.[41] The studies of these patient populations are small, and recommendation for SBRT in these settings should be made only after careful consideration of other options with acknowledgement of the sparsity of outcomes data.
Future Directions
The role of SBRT in localized renal cell carcinoma is actively being defined, and given its excellent short-term local control rates and minimal toxicity, it will likely play a more prominent role in future management. There are several ongoing studies of SBRT as an alternative to surgery in localized renal cell carcinoma, which should add to the currently limited body of evidence supporting its use. One phase II study is evaluating renal impairment in 30 inoperable renal cell carcinoma patients treated with SBRT at 27.5 to 40 Gy in 5 fractions (ClinicalTrials.gov identifier: NCT03747133). Another study plans to enroll 30 inoperable patients to evaluate quality of life after SBRT (35 to 40 Gy in 5 fractions), as well as cost effectiveness (ClinicalTrials.gov identifier: NCT03108703). The ARREST study will treat a similar cohort of 30 patients with SBRT (35 Gy in 5 fractions) and assess for toxicity as a primary endpoint (ClinicalTrials.gov identifier: NCT02853162). FASTRACK II, the largest ongoing phase II study, plans to treat 70 renal cell carcinoma patients with SBRT-either 26 Gy in 1 fraction (if < 4 cm) or 42 Gy in 3 fractions (if > 4 cm), with a primary outcome of freedom from local progression assessed by Response Evaluation Criteria in Solid Tumors (RECIST; ClinicalTrials.gov identifier: NCT02613819). Another phase II study plans to enroll 16 inoperable patients and treat them with SBRT to 36 Gy in 3 fractions, 40 Gy in 4 fractions, or 40 Gy in 5 fractions, to evaluate local control (ClinicalTrials.gov identifier: NCT02141919).
Current studies are also evaluating the efficacy of renal SBRT in combination with other local or systemic therapies. A phase I dose-escalation study is ongoing to evaluate SBRT doses of 30, 40, and 50 Gy in 5 fractions followed by microwave ablation, with a primary outcome of dose-limiting toxicity (ClinicalTrials.gov identifier: NCT02782715). An area of particular interest is the potential for a synergistic effect with SBRT and concomitant immunotherapy. Recent studies have demonstrated the effectiveness of SBRT and immunotherapy in mouse models of renal cell carcinoma,[42] specifically that SBRT has immunomodulatory effects in patients undergoing cytoreductive nephrectomy after SBRT.[43] In one mouse model of renal cell carcinoma, investigators showed that the combination of ablative radiotherapy and programmed death 1 (PD-1) blockade induced near-complete regression of the irradiated primary tumor, as compared with radiation alone or radiation plus a control antibody. There was also a significant abscopal effect, with more than 50% reduction in the size of nonirradiated secondary tumors.[42] Another group analyzed the immunomodulatory effects of SBRT in renal cell carcinoma by treating patients with metastatic disease with SBRT to the primary tumor followed by cytoreductive nephrectomy. They showed that SBRT-treated tumors had increased calreticulin and tumor-associated antigen expression. In addition, they found increased proliferating CD8+ T cells and FOXP3+ cells in the SBRT-treated tumors compared with controls that received no neoadjuvant SBRT. These studies, and others, have led to the many active clinical trials evaluating the efficacy of immunotherapy and other biologic agents in combination with SBRT, both published[41] and yet unpublished (ClinicalTrials.gov identifiers: NCT01896271, NCT02781506, NCT02306954, and NCT02334709).
Discussion/Conclusion
The current state of the literature demonstrates the efficacy and tolerability of renal SBRT in patients with nonoperative renal cell carcinoma, though studies are early-phase and limited by small cohort sizes and lack of long-term follow-up for toxicities and outcomes. Thus, surgery remains the standard of care for localized renal cell carcinoma. However, in nonoperative candidates, SBRT can be offered in a single fraction of 26 Gy; 3 fractions to a total dose of 36 to 45 Gy; or 5 fractions to a total dose of 40 to 50 Gy. The high rates of local control reported in the prospective studies to date suggest that renal SBRT has the potential to impact outcomes for nonsurgical patients. In addition, studies evaluating SBRT in combination with immunotherapy or targeted agents may improve outcomes for patients with more advanced disease. Thus, SBRT is a reasonable treatment option in the current management of nonsurgical patients with localized renal cell carcinoma, and ongoing and future studies will better define its place in multimodality management.
KEY POINTS
• Single-institution prospective studies have shown high rates of local control and acceptable rates of toxicity when renal stereotactic body radiation therapy (SBRT) is employed in nonoperative candidates with renal cell carcinoma.
• Longer follow-up is needed to evaluate long-term outcomes and toxicities after renal SBRT.
• Renal SBRT can be considered for select patients with localized renal cell carcinoma who are not candidates for surgery.
• Further prospective studies are necessary to better define the role of SBRT in localized renal cell carcinoma.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any service or provider of any product mentioned in this article.
PERSPECTIVE

Local Control Is Not the Only Outcome of Interest in RCC
Viraj Master, MD, PhD, FACS
The authors of the accompanying article are to be congratulated for a highly detailed and informative review of a fast-evolving concept in the care of kidney cancer patients, namely the role of radiation therapy in localized kidney cancer. The role of radiation therapy for the treatment of symptomatic kidney cancer metastases is well established, and most modern approaches favor higher-fraction, shorter-duration treatments, which is certainly favored by both patients and their caregivers. At our very high-volume kidney cancer center, I routinely refer all of my patients with symptomatic kidney cancer metastases for site-directed treatment utilizing either radiosurgery or percutaneous approaches. The benefit is clear.
However, with regard to localized kidney cancer, the gold-standard curative therapy is currently surgery. The field has moved to active surveillance for many patients who are not candidates for surgical treatment. The data supporting active surveillance are extensive, with long-term studies showing that active surveillance of small renal masses is associated with excellent survival. In this review, the authors correctly observe that the local control of kidney masses after radiation is excellent. Further, they provide guidance on dose, as well as important observations on technical aspects of dose delivery, including the use of fiducial markers-which are especially important given the significant displacement of the kidney with normal respiration.
However, local control, if measured purely by size, is not an appropriate measure. After radiotherapy, is the kidney cancer dead or not? Control of growth itself is not the oncologic outcome of interest per se. It is notable that the most recent publication cited by the authors demonstrates a 100% local control rate, but the development of distant metastases in 11% of patients.[1] If radiotherapy was working to kill the tumor, this number compares unfavorably to active surveillance. For a population of patients who have essentially T1 tumors, this is higher than expected, even among patients who I would counsel regarding active surveillance. The ideal trial would randomize patients to active surveillance or radiotherapy, with both local and distant endpoints, in addition to quality-of-life endpoints.
Renal function after radiation must be considered, as the authors point out. Radiation to the kidneys produces modest declines in kidney function, which is very important information. But, clinically, many patients experience declines in kidney function over time, particularly those with highly compromised kidney function. Patients who have stage 4 or 5 chronic kidney disease may not be able to tolerate the small declines in kidney function associated with radiation therapy.
As it currently stands, radiation therapy for localized kidney cancer should be considered only in the context of clinical trials, particularly prospective trials. In my humble opinion, the ideal study would be prospective in nature, evaluating patients with biopsy-proven, high-grade kidney cancer that is growing, meaning it meets the criteria for treatment. As the field learns more from genetic studies, such as the TRACERx Renal trial,[2] we will be able to distinguish lethal tumors from indolent tumors. Many times, the indolent tumors are found in older, frail patients with comorbidities.
Possibly the most exciting prospect of radiation therapy, for which the authors detail a number of clinical trials in progress, is its immunomodulatory role, with or without systemic therapy agents, in the treatment of patients with kidney cancer. Multimodal therapy for renal cancer, including radiation therapy, may be important. Our field needs to design the appropriate trials to prove it.
Financial Disclosure:Dr. Master has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
REFERENCES
1. Siva S, Louie AV, Warner A, et al. Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: a report from the International Radiosurgery Oncology Consortium for Kidney (IROCK). Cancer. 2018;124:934-42.
2. Turajlic S, Xu H, Litchfield K, et al; PEACE; TRACERx Renal Consortium. Tracking cancer evolution reveals constrained routes to metastases: TRACERx Renal. Cell. 2018;173:581-94.e12.
Dr. Master is a Professor, Associate Chair for Clinical Affairs and Quality, and Director of the Clinical Research Unit in the Department of Urology at Emory University in Atlanta, Georgia.
References:
1. van der Werf-Messing B. Proceedings: carcinoma of the kidney. Cancer. 1973;32:1056-61.
2. Juusela H, Malmio K, Alfthan O, Oravisto KJ. Preoperative irradiation in the treatment of renal adenocarcinoma. Scand J Urol Nephrol. 1977;11:277-81.
3. Finney R. The value of radiotherapy in the treatment of hypernephroma--a clinical trial. Br J Urol. 1973;45:258-69.
4. Kjaer M, Iversen P, Hvidt V, et al. A randomized trial of postoperative radiotherapy versus observation in stage II and III renal adenocarcinoma. A study by the Copenhagen Renal Cancer Study Group. Scand J Urol Nephrol. 1987;21:285-9.
5. Deschavanne PJ, Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys. 1996;34:251-66.
6. Cochran DC, Chan MD, Aklilu M, et al. The effect of targeted agents on outcomes in patients with brain metastases from renal cell carcinoma treated with Gamma Knife surgery. J Neurosurg. 2012;116:978-83.
7. Kim YH, Kim JW, Chung HT, et al. Brain metastasis from renal cell carcinoma. Prog Neurol Surg. 2012;25:163-75.
8. Staehler M, Haseke N, Nuhn P, et al. Simultaneous anti-angiogenic therapy and single-fraction radiosurgery in clinically relevant metastases from renal cell carcinoma. BJU Int. 2011;108:673-8.
9. Kothari G, Foroudi F, Gill S, et al. Outcomes of stereotactic radiotherapy for cranial and extracranial metastatic renal cell carcinoma: a systematic review. Acta Oncol. 2015;54:148-57.
10. Balagamwala EH, Angelov L, Koyfman SA, et al. Single-fraction stereotactic body radiotherapy for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2012;17:556-64.
11. Jhaveri PM, Teh BS, Paulino AC, et al. A dose-response relationship for time to bone pain resolution after stereotactic body radiotherapy (SBRT) for renal cell carcinoma (RCC) bony metastases. Acta Oncol. 2012;51:584-8.
12. Zelefsky MJ, Greco C, Motzer R, et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:1744-8.
13. Svedman C, Sandström P, Pisa P, et al. A prospective phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol. 2006;45:870-5.
14. Teh B, Bloch C, Galli-Guevara M, et al. The treatment of primary and metastatic renal cell carcinoma (RCC) with image-guided stereotactic body radiation therapy (SBRT). Biomed Imaging Interv J. 2007;3:e6.
15. Corbin KS, Ranck MC, Hasselle MD, et al. Feasibility and toxicity of hypofractionated image guided radiation therapy for large volume limited metastatic disease. Pract Radiat Oncol. 2013;3:316-22.
16. Walsh L, Stanfield JL, Cho LC, et al. Efficacy of ablative high-dose-per-fraction radiation for implanted human renal cell cancer in a nude mouse model. Eur Urol. 2006;50:795-800; discussion 800.
17. Chang JH, Cheung P, Erler D, et al. Stereotactic ablative body radiotherapy for primary renal cell carcinoma in non-surgical candidates: initial clinical experience. Clin Oncol (R Coll Radiol). 2016;28:e109-14.
18. Kaidar-Person O, Price A, Schreiber E, et al. Stereotactic body radiotherapy for large primary renal cell carcinoma. Clin Genitourin Cancer. 2017;15:e851-4.
19. Lo CH, Huang WY, Chao HL, et al. Novel application of stereotactic ablative radiotherapy using CyberKnifeî for early-stage renal cell carcinoma in patients with pre-existing chronic kidney disease: initial clinical experiences. Oncol Lett. 2014;8:355-60.
20. Sun MR, Brook A, Powell MF, et al. Effect of stereotactic body radiotherapy on the growth kinetics and enhancement pattern of primary renal tumors. AJR Am J Roentgenol. 2016;206:544-53.
21. Staehler M, Bader M, Schlenker B, et al. Single fraction radiosurgery for the treatment of renal tumors. J Urol. 2015;193:771-5.
22. Siva S, Jackson P, Kron T, et al. Impact of stereotactic radiotherapy on kidney function in primary renal cell carcinoma: establishing a dose-response relationship. Radiother Oncol. 2016;118:540-6.
23. McBride SM, Wagner AA, Kaplan ID. A phase 1 dose-escalation study of robotic radiosurgery in inoperable primary renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2013;87:S84.
24. Ponsky L, Lo SS, Zhang Y, et al. Phase I dose-escalation study of stereotactic body radiotherapy (SBRT) for poor surgical candidates with localized renal cell carcinoma. Radiother Oncol. 2015;117:183-7.
25. Kaplan ID, Redrosa I, Martin C, et al. Results of a phase I dose escalation study of stereotactic radiosurgery for primary renal tumors. Int J Radiat Oncol Biol Phys. 2010;78:S191.
26. Pham D, Thompson A, Kron T, et al. Stereotactic ablative body radiation therapy for primary kidney cancer: a 3-dimensional conformal technique associated with low rates of early toxicity. Int J Radiat Oncol Biol Phys. 2014;90:1061-8.
27. Siva S, Pham DJ, Tan TH, et al. Principal analysis of a phase Ib trial of stereotactic body radiation therapy (SBRT) for primary kidney cancer. Int J Radiat Oncol Biol Phys. 2016;96:S96.
28. Siva S, Pham D, Kron T, et al. Stereotactic ablative body radiotherapy for inoperable primary kidney cancer: a prospective clinical trial. BJU Int. 2017;120:623-30.
29. Siva S, Ellis RJ, Ponsky L, Teh BS, Mahadevan A, Muacevic A, Staehler M, Onishi H, Wersall P, Nomiya T, Lo SS: Consensus statement from the international radiosurgery oncology consortium for kidney for primary renal cell carcinoma. Future Oncol 2016;12:637-645.
30. Wang YJ, Han TT, Xue JX, Chang DS, Li HQ, Li P, Wang JD, Wang YJ, Xia TY: Stereotactic gamma-ray body radiation therapy for asynchronous bilateral renal cell carcinoma. Radiol Med 2014;119:878-883.
31. Siva S, Pham D, Gill S, Corcoran NM, Foroudi F: A systematic review of stereotactic radiotherapy ablation for primary renal cell carcinoma. BJU Int 2012;110:E737-743.
32. Siva S, Pham D, Gill S, Bressel M, Dang K, Devereux T, Kron T, Foroudi F: An analysis of respiratory induced kidney motion on four-dimensional computed tomography and its implications for stereotactic kidney radiotherapy. Radiat Oncol 2013;8:248.
33. Sonier M, Chu W, Lalani N, Erler D, Cheung P, Korol R: Evaluation of kidney motion and target localization in abdominal sbrt patients. J Appl Clin Med Phys 2016;17:429-433.
34. Pham D, Kron T, Bressel M, Foroudi F, Hardcastle N, Schneider M, Soteriou S, Innes J, Siva S: Image guidance and stabilization for stereotactic ablative body radiation therapy (sabr) treatment of primary kidney cancer. Practical radiation oncology 2015;5:e597-605.
35. Siva S, Louie AV, Warner A, Muacevic A, Gandhidasan S, Ponsky L, Ellis R, Kaplan I, Mahadevan A, Chu W, Swaminath A, Onishi H, Teh B, Correa RJ, Lo SS, Staehler M: Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: A report from the international radiosurgery oncology consortium for kidney (irock). Cancer 2018;124:934-942.
36. Yamamoto T, Kadoya N, Takeda K, Matsushita H, Umezawa R, Sato K, Kubozono M, Ito K, Ishikawa Y, Kozumi M, Takahashi N, Katagiri Y, Onishi H, Jingu K: Renal atrophy after stereotactic body radiotherapy for renal cell carcinoma. Radiat Oncol 2016;11:72.
37. Jackson P, Foroudi F, Pham D, Hofman MS, Hardcastle N, Callahan J, Kron T, Siva S: Short communication: Timeline of radiation-induced kidney function loss after stereotactic ablative body radiotherapy of renal cell carcinoma as evaluated by serial (99m)tc-dmsa spect/ct. Radiat Oncol 2014;9:253.
38. Muller AC, van Oorschot B, Micke O, Guckenberger M: [german s3 guideline for renal cell carcinoma : Presentation and discussion of essential aspects for the radiation oncologist]. Strahlenther Onkol 2018;194:1-8.
39. Correa RJM, Ahmad B, Warner A, Johnson C, MacKenzie MJ, Pautler SE, Bauman GS, Rodrigues GB, Louie AV: A prospective phase i dose-escalation trial of stereotactic ablative radiotherapy (sabr) as an alternative to cytoreductive nephrectomy for inoperable patients with metastatic renal cell carcinoma. Radiation oncology (London, England) 2018;13:47-47.
40. Frick MA, Chhabra AM, Lin L, Simone CB, 2nd: First ever use of proton stereotactic body radiation therapy delivered with curative intent to bilateral synchronous primary renal cell carcinomas. Cureus 2017;9:e1799.
41. Svedman C, Karlsson K, Rutkowska E, Sandstrom P, Blomgren H, Lax I, Wersall P: Stereotactic body radiotherapy of primary and metastatic renal lesions for patients with only one functioning kidney. Acta Oncol 2008;47:1578-1583.
42. Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP, Mansfield AS, Furutani KM, Olivier KR, Kwon ED: Pd-1 restrains radiotherapy-induced abscopal effect. Cancer immunology research 2015;3:610-619.
43. Singh AK, Winslow TB, Kermany MH, Goritz V, Heit L, Miller A, Hoffend NC, Stein LC, Kumaraswamy LK, Warren GW, Bshara W, Odunsi K, Matsuzaki J, Abrams SI, Schwaab T, Muhitch JB: A pilot study of stereotactic body radiation therapy combined with cytoreductive nephrectomy for metastatic renal cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research 2017;23:5055-5065.
