Sarcoidosis in a Man With Renal Carcinoid Tumor
The patient is a 39-year-old Caucasian male who presented with a right renal mass and painless gross hematuria. He underwent a right laparoscopic radical nephrectomy and the final pathology revealed a carcinoid tumor.
SECOND OPINION
Multidisciplinary Consultations on Challenging Cases
The University of Colorado Denver School of Medicine faculty holds weekly second opinion conferences focusing on cancer cases that represent most major cancer sites. Patients seen for second opinions are evaluated by an oncologic specialist. Their history, pathology, and radiographs are reviewed during the multidisciplinary conference, and then specific recommendations are made. These cases are usually challenging, and these conferences provide an outstanding educational opportunity for staff, fellows, and residents in training.
The second opinion conferences include actual cases from genitourinary, lung, melanoma, breast, neurosurgery, gastrointestinal, and medical oncology. On an occasional basis,
ONCOLOGY
will publish the more interesting case discussions and the resultant recommendations. We would appreciate your feedback; please contact us at
second.opinion@uchsc.edu
.
E. David Crawford, MD
Al Barqawi, MD
Guest Editors
University of Colorado Health Sciences Center
and Univeristy of Colorado Cancer Center
Denver, Colorado
The patient is a 39-year-old Caucasian male who presented with a right renal mass and painless gross hematuria. He underwent a right laparoscopic radical nephrectomy and the final pathology revealed a carcinoid tumor (5.5 cm; T1b, Nx, Mx). The postoperative course was unremarkable. Approximately 3 months postop, he developed bilateral hilar adenopathy. No clinical evidence of carcinoid or sarcoid symptoms were observed.
History, Physical Exam, and Laboratory Findings
Dr. Fernando Kim: The patient originally presented with a right-sided flank pain and hematuria. Radiographic evaluation demonstrated a 5.5-cm right kidney mass. The patient was referred to the Tony Grampsas Cancer Center, Urology Clinic in Denver for evaluation of this mass and hematuria.
Cystoscopy and cytology were negative for malignancy. The patient is an avid skier and had no other medical problems. He had a right shoulder surgery 4 years ago in addition to a thoracic compression fracture without surgical repair, both related to sporting accidents. Although the patient did not have a family history of kidney cancer, his grandfather had prostate cancer.
Complete physical exam was unremarkable. Work-up for metastasis included axial computerized tomography (CT) of the abdomen, pelvis, and chest with and without IV contrast, since his creatinine and liver function tests were within normal reference ranges. Management options included laparoscopic and/or open radical nephrectomy vs partial nephrectomy.
Surgical Management
Dr. Kim: What is your view of a partial nephrectomy vs a radical nephrectomy in this patient?
Dr. E. David Crawford: The indications for a partial nephrectomy have expanded in the past decade. Earlier, the indication for partial resection was relatively narrow, including, for example, a solitary kidney or impaired renal function. A tumor size less than 4 to 5 cm was also a necessity. Experience now suggests that results with partial nephrectomy are comparable to radical nephrectomy in properly selected individuals.
Dr. Kim: What is the role of laparoscopic surgery vs an open approach in this case?
Dr. Crawford: In most cases, a laparoscopic approach inflicts less morbidity on the patient. The results are closely correlated with proper patient selection and the expertise of the laparoscopic surgeon.
Dr. Shandra Wilson: In addition, patients who may be at higher risk with a laparoscopic procedure vs open nephrectomy are (1) those requiring a partial nephrectomy who do not have an experienced laparoscopic surgeon (tumors that are not central and are generally smaller than 4 to 5 cm), (2) those with possible xanthogranulomatous pyelonephritis, and (3) those with a thrombus of the renal vein or vena cava involvement. A clear benefit exists for a laparoscopic approach in patients with metastatic disease and a limited life expectancy, who will benefit even more from a shorter recovery period after surgery. Robotic assistance has added to the ability to resect these more difficult tumors using a laparoscopic approach.
Dr. Kim: The patient consented to surgery, and a laparoscopic, adrenal-sparing nephrectomy was performed. The traditional transperitoneal laparoscopic radical nephrectomy using a four-trocars technique was performed. After insufflation of the abdomen with CO2 using a Veress needle, the trocars were placed under direct visualization and the colon was kocherized, exposing the kidney and allowing control of vessels and ureter without violating the tumor, as described previously by Kim et al.[1]
The patient's American Society of Anesthesiologists (ASA) score was 2; body mass index, 26.3; estimated blood loss, less than 50 mL; and total operating time, 1 hour and 40 minutes.
The patient was discharged home and his follow-up revealed no signs or symptoms of trocar site herniation. Due to the unusual pathology, I ordered an octreotide scan plus CT scans of the chest, abdomen, and pelvis.
Pathology
FIGURE 1
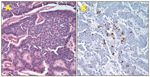
Renal Cell Tumor
Dr. Francisco G. La Rosa: We received a right kidney with a 5.5-cm maximal diameter tumor originating in the area of the medulla and extending into the hilum of the kidney. Microscopic examination revealed renal parenchyma invaded with a tumor composed of nests of uniform cells with rounded uniform nuclei, conspicuous nucleoli, and eosinophilic cytoplasm with some cells arranged in a gland-like fashion (Figure 1A). The tumor showed focal extension into the hilar fat with no evidence of perivesicular or angiolymphatic invasion; the resection margins were negative for tumor. Immunoperoxidase staining showed the tumor cells to be focally positive for chromogranin (Figure 1B) and weakly positive for CD56 antigen.
Dr. Kim: Due to the new findings on follow-up imaging studies, the patient underwent mediastinoscopy for lymph node sampling approximately 3 months after his laparoscopic radical nephrectomy. Let us review these pathology findings.
FIGURE 2
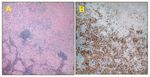
Mediastinal Lymph Node
Dr. La Rosa: The right, precarinal, and subcarinal mediastinal lymph nodes showed marked architectural effacement by a population of histiocytes (Figure 2A). All specimens showed nonnecrotizing granulomatous inflammation with extensive sclerosis. The identity of the histiocytes was confirmed by their reactivity for CD68 antigen (Figure 2B). They were negative for S-100 protein, synaptophysin, HMB-45, pan-cytokeratin AE1/3, and carcinoembryonic antigen. Special stains for acid-fast bacilli and a silver stain for fungi revealed no microorganisms. We have reviewed this case with Dr. John Ryder, a hematopathologist from our department, and with Dr. Kenneth A. Iczkowski, the pathologist who originally reviewed this case, and our consensus is that the findings are consistent with sarcoidosis.
Radiology
Dr. Kim: The initial magnetic resonance imaging (MRI) scan of the abdomen was requested by the primary care physician.
FIGURE 3
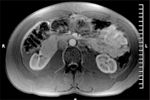
MRI Scan of the Abdomen
Radiology: The main finding on magnetic resonance imaging is an enhancing soft-tissue mass measuring 5 x 3.8 cm in the anterior portion of the right kidney (Figure 3). Additionally, there is a 2 x 1.5-cm complex cystic structure inferior to the mass. The right main renal artery, renal vein, and inferior vena cava appear patent.
Dr. Kim: Let us review the scans of the mediastinal mass.
FIGURE 4
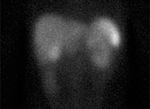
Octreotide Scan of the Chest
Radiology: A 24-hour status postradionuclide administration of 5.3 μCi indium-111 pentetreotide (OctreoScan IV), whole-body anterior/posterior imaging was obtained. Single-photon emission computed tomography (SPECT) imaging of the body from the chest, abdomen, and pelvis was also obtained and reviewed in the transaxial, coronal, and sagittal projections. The chest showed nodular foci of mildly to moderately increased radiotracer uptake in both pulmonary hilar regions (Figure 4). This is suspicious for metastatic adenopathy, as physiologic uptake is not normally visualized in lymph nodes. The lung parenchyma is negative for abnormally increased uptake.
These findings are suspicious for bilateral hilar metastatic adenopathy, although the uptake in this region is mildly increased, relative to the physiologic uptake seen in the abdominal organs. There is no increased uptake in any other lymph node region. An area of minor uptake is seen in the right-upper quadrant of the abdomen, most likely relating to the gallbladder.
FIGURE 5
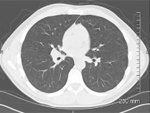
CT Scan of the Chest
Follow-up with a noncontrast CT of the chest revealed lobulated hilar masses, bilaterally, with the right greater than left (Figure 5). Small mediastinal lymph nodes are also noted, although no axillary lymphadenopathy is present.
Medical Management
Dr. Kim: What are the medical management considerations for carcinoid tumor of the kidney?
Dr. Thomas W. Flaig: Primary carcinoid tumors of the kidney are rare,[2] with one recent review describing a total of 56 cases in the English medical literature.[3] The average size of these tumors was 8 cm, with those greater than 4 cm having a worse prognosis than those less than 4 cm. Although not a feature in this case, primary carcinoid of the kidney is associated with horseshoe kidneys and teratoma of the kidneys. The mainstay of treatment and the only curative modality for carcinoid of the kidney is complete surgical resection.
For patients with metastatic carcinoid, several medical interventions are available. Most carcinoid tumors express the somatostatin receptor, and this can be assessed with somatostatin receptor scintigraphy (ie, octreotide scan).[4] The somatostatin analog octreotide is effective in slowing tumor growth and palliating symptoms such as diarrhea and flushing in some cases.[5] However, neuroendocrine syndromes are uncommon in cases of renal carcinoid, with a reported frequency of 12% in the literature.[3] Significant tumor regression with octreotide treatment is uncommon. Patients treated with octreotide may develop side effects such as abdominal discomfort, nausea, and loose stools.
The utility of traditional chemotherapy in treating carcinoid tumors is limited. The efficacy of single-agent therapy has been disappointing, but some combination regimens demonstrate improved activity with response rates of 20% to 30%.[6] One new approach under investigation is the use of radiolabeled somatostatin analogs, which allow for a physical targeting of the somatostatin receptor–expressing tumors with targeted radiation.[7]
In this patient's management, I would recommend upper and lower gastrointestinal endoscopy to rule out another site of carcinoid, since primary kidney carcinoid is rare. Additionally, a serum chromogranin and urinary 5-hydroxyindoleacetic acid (5-HIAA) should be obtained as baseline measurements.
Synchronous Carcinoid and Sarcoid Tumors
Dr. Kim: What is known about the relationship of synchronous carcinoid and sarcoid tumors?
Dr. La Rosa: Carcinoid tumors are low-grade malignant tumors that arise from neuroendocrine cells. These tumors are histologically divided into typical carcinoids, which are considered benign tumors with an excellent prognosis after surgical excision, and atypical carcinoids, considered low-grade malignant tumors with potential for local invasion and distant metastases.
The synchronous appearance of carcinoid and sarcoid tumors is an even more rare event. Certain malignant lesions have the tendency to occur in patients with sarcoidosis, but it is unclear which of these tumors appears first.[8] Only seven patients between the ages of 31 and 66 years with both sarcoidosis and carcinoid tumors have been reported in 44 years at the Mayo Clinic Rochester.[9]
The simultaneous appearance of malignant tumors and sarcoidosis may be explained in one of two ways. First, it appears that some immunologic abnormalities related to sarcoidosis may promote the development of some neoplastic processes. Second, some malignant diseases may promote the onset of sarcoidosis, either by causing a local sarcoid reaction that evolves in a more disseminated fashion or by directly initiating systemic mechanisms to induce sarcoidosis. Because the chronology of events have differed in the cases reported in the literature, various mechanisms of action may have played a role in the manifestations of these two disease entities.
Since carcinoid tumors are neuroendocrine tumors characterized by their ability to express somatostatin receptors, they can be imaged with radiolabeled somatostatin analogs. Based on different patterns of radiotracer activity seen on 111In-pentetreotide and 18F-fluorodeoxyglucose positron-emission tomography (FDG-PET), it is now possible to differentiate regional metastatic carcinoid lymphadenopathy from a sarcoid lymphadenopathy.[10]
The case presented in this Second Opinion discussion, emphasizes the importance of avoiding the diagnosis of disseminated malignant disease in patients with cancer and associated hilar and mediastinal lymphadenopathy before we have the results of a biopsy and pathologic confirmation of metastatic disease. If available, functional imaging studies can complement this differential diagnosis.[10]
Concluding Remarks
Dr. Kim: Renal carcinoid tumors are exceedingly rare tumors that have been primarily documented as case reports in the literature.[2] Primary renal carcinoid tumors seem to be more indolent than renal cell carcinomas, although metastases to regional lymph nodes, liver, and bone have been described. The presence of metastases seems to indicate a more malignant course. However, even with metastases, a patient might live 3 or 4 years.
Renal carcinoid is the second most prevalent genitourinary carcinoid in each sex, following testicular carcinoid tumors in men and ovarian tumors in women. Significant adverse prognostic factors include age greater than 40 years, tumor size greater than 4 cm, purely solid tumors on the cut surface, mitotic rate higher than 1/10 high-power fields, metastasis at initial diagnosis, and tumors extending throughout the renal capsule.
Renal carcinoid tumors should be managed by radical or partial nephrectomy, and in the era of minimally invasive surgery, the laparoscopic approach offers faster recovery, better cosmetics, and less hospitalization-as depicted in this case-without compromising the principles of oncologic surgery. Good outcomes have been reported for organ-confined disease after radical excision. Conventional methods of imaging are inadequate for detecting smaller carcinoid tumors. Thus, somatostatin receptor scintigraphy should complement CT and MRI when searching for occult or metastatic disease. Close follow-up after surgery is necessary.[11]
Financial Disclosure:The participants in this conference have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Kim FJ, Rha KH, Hernandez F, et al: Laparoscopic radical versus partial nephrectomy: Assessment of complications. J Urol 170:408-411, 2003.
2. Daneshmand S, Chandrasoma S, Wilson S: Primary renal carcinoid tumor. Scientific World J 4:378–380, 2004.
3. Romero FR, Rais-Bahrami S, Permpongkosol S, et al: Primary carcinoid tumors of the kidney. J Urol 176:2359-2366, 2006.
4. Mufarrij P, Varkarakis IM, Studeman KD, et al: Primary renal carcinoid tumor with liver metastases detected with somatostatin receptor imaging. Urology 65:1002, 2005.
5. Lamberts SW, van der Lely AJ, de Herder WW, et al: Octreotide. N Engl J Med 334:246-254, 1996.
6. Kulke MH, Mayer RJ: Carcinoid tumors. N Engl J Med 340:858-868, 1999.
7. Kwekkeboom DJ, Teunissen JJ, Bakker WH, et al: Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3] octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol 23:2754-2762, 2005.
8. Weltfriend S, Harth Y, Katz I: Subcutaneous sarcoidosis in a patient with malignant carcinoid tumor of the colon. J Am Acad Dermatol 20:507-508, 1989.
9. Levy NT, Rubin J, DeRemee RA, et al: Carcinoid tumors and sarcoidosis-does a link exist? Mayo Clin Proc 72:112-116, 1997.
10. Avram AM, Mackie GC, Schneider BJ, et al: Differentiation between carcinoid and sarcoid with F-18 FDG PET and In-111 pentetreotide. Clin Nucl Med 31:197-200, 2006.
11. Shurtleff BT, Shvarts O, Rajfer J: Carcinoid tumor of the kidney: Case report and review of the literature. Rev Urol 7:229-233, 2005.