Sequential Use of XELOX and XELIRI May Allow Greater Use of Oxaliplatin in Patients With Metastatic Colorectal Cancer
GUADALAJARA, SPAIN-Sequentialcycling of XELOX (capecitabine[Xeloda]/oxaliplatin [Eloxatin])and XELIRI (capecitabine/irinotecan
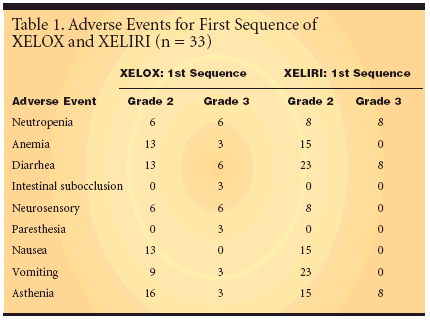
GUADALAJARA, SPAIN-Sequentialcycling of XELOX (capecitabine[Xeloda]/oxaliplatin [Eloxatin])and XELIRI (capecitabine/irinotecan[Camptosar]) provides good tumorcontrol with a low rate of grade 3neurosensory toxicity in patients withmetastatic colorectal cancer, accordingto preliminary results of a phase IImulticenter trial (abstract 3544).The investigators, who specificallywanted to evaluate the impact of sequentialcycling on the dose-limitingneurosensory toxicity associated withoxaliplatin, reported that "the low rateof grade 3 neurosensory toxicity ispromising." Among the 33 enrolledpatients, there were six cases each ofgrade 2 and 3 neurosensory adverseevents in the group completing thefirst sequence of XELOX and eightgrade 2 but no grade 3 adverse eventsin the group completing the first sequenceof XELIRI. More commonadverse events were anemia, diarrhea,nausea, vomiting, and asthenia (Table1).Same Capecitabine DoseThe XELOX treatment regimen forthe ongoing study is oral capecitabine(1,000 mg/m2 twice daily on days 1 to14) with IV oxaliplatin (130 mg/m2 onday 1). This is followed by the XELIRIregimen, which consists of the samedose of capecitabine but with 240mg/m2 of IV irinotecan on day 1.Thirty-two patients had completedthe first sequence of XELOX, with13 going on to complete XELIRI. Leadinvestigator Javier Cassinello, MD, ofHospital General in Guadalajara,Spain, reported that he expected mostof those 32 patients will also completethe first cycle of XELIRI. Three patientscompleted the second sequenceof XELOX, with two going on to completethe second sequence of XELIRI.Stop-and-Go TreatmentDr. Cassinello explained that theaim of the study was to test a "stopand-go treatment" with four cycles ofXELOX, then stopping treatment andresuming with four cycles of XELIRI."Then, if you can, restart treatmentwith oxaliplatin, knowing the neurosensorytoxicity is coming down andyou can start again with 100% of theoxaliplatin dose." This should allowthe patient to receive more first-linetreatment with oxaliplatin, which theinvestigators noted is the only platinumcompound with clinical activityagainst metastatic colorectal cancer.The median age of patients was 69(range 41-78 years). The Eastern CooperativeOncology Group (ECOG)status was 0 for 33% of patients and ≥1 for 67%. The primary tumor site wasthe colon for 67% of patients, the rectumfor 30%, and both sites for 3% ofpatients. Most patients (61%) hadstage IV disease.Overall Response of 47%The overall response rate, based onintent-to-treat analysis, was 47% (95%confidence interval [CI]: 24.9%-69.9%). That rate includes one patientwith a complete response and eightpatients with partial responses. Six patientshad stable disease and four patientshad progressive disease. Thirteenpatients were not evaluable dueto adverse events, loss of follow-up, orbecause treatment was ongoing.
The median time to disease progressionwas 11.9 months (95% CI:4.4-19.5). The median overall survivalhad not yet been reached at the time ofthis report.