Studies Help Refine Anticancer Activity, Unravel Drug Resistance
BETHESDA, Maryland-An understanding of how camptothecins intrude between topoisomerase I and DNA is helping to refine the anticancer activity of these drugs, and studies of the pathway from initial camptothecin binding to final cell death may help unravel the mechanisms behind drug resistance. Yves Pommier, MD, PhD, chief of the National Cancer Institute’s Laboratory of Molecular Pharmacology reviewed recent developments in these areas at the Vanderbilt University Symposium.
BETHESDA, MarylandAn understanding of how camptothecins intrude between topoisomerase I and DNA is helping to refine the anticancer activity of these drugs, and studies of the pathway from initial camptothecin binding to final cell death may help unravel the mechanisms behind drug resistance. Yves Pommier, MD, PhD, chief of the National Cancer Institute’s Laboratory of Molecular Pharmacology reviewed recent developments in these areas at the Vanderbilt University Symposium.
DNA topoisomerase inhibitors are among the most commonly used anticancer and antibacterial drugs, Dr. Pommier noted. Camptothecins are topoisomerase I inhibitors. "Topoisomerases can also be inhibited by DNA damage and by chemotherapeutic alterations of DNA, such as DNA strand breaks, abasic sites, mismatches, and base damage," he said.
Where Camptothecins Bind
Dr. Pommier explained that camptothecins all work in basically the same wayby interfering with DNA religation through production of a cleavable topoisomerase I-camptothecin complex. The basic camptothecin structure presents many potential sites for creation of new derivatives, and several of these are under active clinical study.
Understanding where camptothecins bind in the topoisomerase I-DNA complex is important for understanding and perhaps refining their mechanism of action, Dr. Pommier noted. This question has been explored through analysis of topoisomerase I mutations that lead to camptothecin resistance.
"These mutations are clustered at the enzyme-DNA interface," Dr. Pommier said. These studies have enabled researchers to construct an image of exactly how camptothecin fits into the topoisomerase I-DNA complex, which in turn has suggested new targets for new drug development.
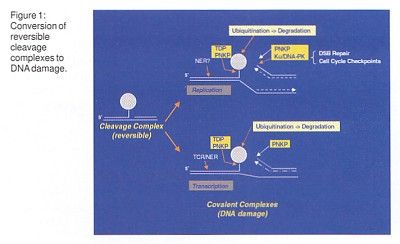
Once this docking occurs, reversible topoisomerase I cleavage complexes are converted to permanent DNA damage by replication fork collisions. These can occur either during replication or during transcription and involve ubiquitination (binding to ubiquitin) and degradation of the DNA (Figure 1).
The time course of the resulting apoptosis varies depending on tumor type. Recent studies of camptothecin effects in different tumor cell lines point to caspase dependence and requirement of protein synthesis as important factors in delaying cell death in some treated cancers.
"Protein synthesis and caspase activity are required for camptothecin-induced DNA fragmentation in HT29 colorectal cancer cells," Dr. Pommier said. Delayed apoptosis in these cells is due to delayed activation of caspase-3 and to degradation of poly (ADP-ribose) polymerase induced by camptothecin.
Fas and Fas-ligand (Fas-L) also come into play. Dr. Pommier said that after treatment with camptothecin, there is an accumulation of FasL and of Fas just before onset of apoptosis in colorectal cancer cell lines. Camptothecins also affect expression of p53, which mediates production of other proteins important in the apoptotic response, such as BAX, Bcl-X2, and p21.
"Fas-dependent cell killing induced by camptothecins is independent of Fas ligand," Dr. Pommier said. "We now believe that camptothecin-induced apoptosis, at least in HT29 colorectal cancer cells, involves the following pathway: Camptothecin produces DNA damage mediated by topoisomerase I. This leads to alterations in macromolecular synthesis of BAX, Fas, and perhaps Fas ligand. The upregulation of BAX and Fas activate caspase. The result is apoptosis."