Thalidomide Demonstrates Multiple Roles in Myeloma
SAN FRANCISCO-Studies presented at the 42nd Annual Meeting of the American Society of Hematology (ASH) show that thalidomide (Thalomid) has value as a single agent in treating advanced and refractory myeloma and that thalidomide combined with dexamethasone is useful in treating resistant and newly diagnosed disease.
SAN FRANCISCOStudies presented at the 42nd Annual Meeting of the American Society of Hematology (ASH) show that thalidomide (Thalomid) has value as a single agent in treating advanced and refractory myeloma and that thalidomide combined with dexamethasone is useful in treating resistant and newly diagnosed disease.
Major Single-Agent Activity
Long-term follow-up of 169 patients in a phase II trial indicates that single-agent thalidomide has major activity in advanced and refractory multiple myeloma: 36% of patients achieved at least a 25% reduction in myeloma protein. The projected 2-year survival is approximately 60%, with about 20% remaining event-free, reported Bart Barlogie, MD, PhD, of the Myeloma and Transplant Research Center, University of Arkansas for Medical Sciences, Little Rock.
"As we don’t know the mechanism by which thalidomide brings about its remarkable activity, we did examine the prognostic features associated with outcome," Dr. Barlogie said. Adverse prognostic features included the presence of abnormal cytogenetics, plasma cell labeling index (PCLI) greater than 0.5%, and an elevated beta-2 microglobulin (B2M) (greater than 3 mg/L).
About two thirds of the patients in the study had abnormal cytogenetics, including one third with chromosome 13 deletion, "which we now recognize as one of the most adverse features for outcome in this disease," Dr. Barlogie said.
Thirty patients with no adverse risk factors had a very good outcome (see Figure). "At 30 months, 90% of these patients are projected to be alive and more than 40% still event-free," Dr. Barlogie said.
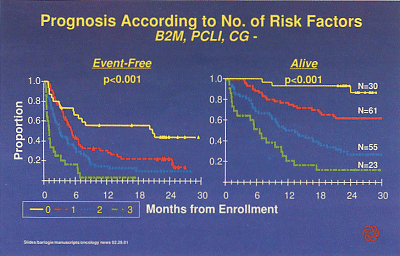
Figure 1 shows event-free and overall survival according to number of risk factors among 169 patients with advanced and refractory multiple myeloma treated with single-agent thalidomide. The phase II trial was conducted by Dr. Bart Barlogie, of the University of Arkansas for Medical Sciences, Little Rock. Prognostic factors included plasma cell labeling index (PCLI), elevated beta-2 microglobulin (B2M), and abnormal cytogenetics (CG-).
Patients were started on thalidomide at 200 mg/d "with doses escalated by 200 mg increments, according to tolerance, to a maximum of 800 mg, so that this far-advanced patient population could maximally benefit," Dr. Barlogie said.
Dose a Factor
At 3 months, Dr. Barlogie asked whether patients surviving this landmark had received more than the median thalidomide dose (42 g in 3 months, or 400 mg/d).
"The response rate was better in those patients who had the higher dose equivalent, regardless of where one cut the response levelat 25%, 50%, or more than 75% reduction in myeloma protein," Dr. Barlogie said. "This was also true for overall survival, but not for event-free survival. Those patients who made it through the 3-month landmark and had received more than 42 g in 3 months had a superior outcome."
The Next Step
A logical next step in evaluating thalidomide is the investigation of its potential contribution in the up-front management of newly diagnosed patients. In Dr. Barlogie’s current study, such patients are randomized up front to "Total Therapy II" with or without thalidomide 400 mg.
Total Therapy II, as used by Dr. Barlogie at Arkansas, employs four phases of therapy:
induction with VAD (vincristine, Adriamycin, dexamethasone), DCEP#1 (dexamethasone, cyclophosphamide, etoposide, cisplatin), CAD (cyclophosphamide, Adriamycin, dexamethasone) with peripheral blood stem cell collection, and DCEP#2
tandem transplant with melphalan (Alkeran) 100 mg/m2
consolidation with DCEP or DCEP/CAD for 1 year
interferon maintenance.
Early results showed a significant increase in the incidence of complete and near-complete responses, up to 70% after two transplants, regardless of treatment arm (Figure 2), Dr. Barlogie said.
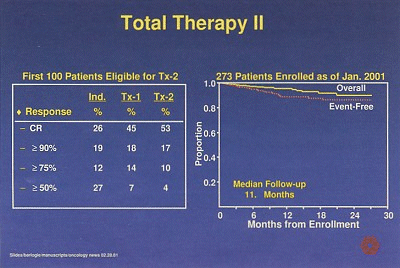
Figure 2 shows response rates (CR = complete response) in the first 100 patients and survival (273 patients) in a randomized study of Total Therapy II (Tx-2) with or without thalidomide among patients with newly diagnosed multiple myeloma. The charts include patients from both arms of the study, conducted by Dr. Bart Barlogie, of the University of Arkansas for Medical Sciences, Little Rock.
"Unprecedented 2-year event-free and overall survival rates of 87% and 91%, respectively, have been noted among the 273 patients enrolled as of December 2000, with a median follow-up of 12 months," he said. It is anticipated that randomization data will be available in about 1 year.
Synergy Suggested
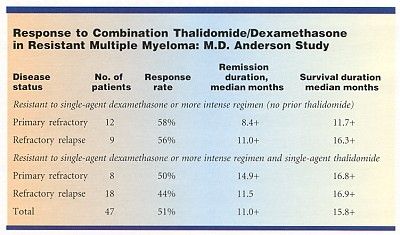
The combination of thalidomide-dexamethasone showed "superior activity," compared with thalidomide alone, in a study involving 47 patients with resistant multiple myeloma, said Donna M. Weber, MD, assistant professor, Department of Lymphoma and Myeloma, M.D. Anderson Cancer Center.
The combination was effective in 46% of 26 patients who were previously resistant to both single-agent thalidomide and single-agent dexamethasone, suggesting synergy, she said (see Table). In addition, the rapid time to response may be suggestive of a direct antitumor effect.
Patients received 200 mg of thalidomide daily, which was increased (in the absence of severe side effects) in 200 mg increments to a maximum dose of 800 mg. In addition, 20 mg/m2 of dexamethasone was administered on days 1 to 5 and days 15 to 18. Those responding were maintained on a maximally tolerated dose of thalidomide and dexamethasone on days 1 to 5 each month.
Partial response, defined as greater than 50% reduction in serum myeloma protein and/or more than 75% reduction in Bence-Jones protein, occurred in 24 patients (52%). Similar response rates were seen in patients with primary refractory and refractory relapsed disease. The projected median remission is more than 10 months.
Dr. Weber said that the side effects were generally similar to those previously reported for thalidomide and dexamethasone alone or in combination.
Longer follow-up, however, unmasked more frequent neuropathy (43%), which was sometimes irreversible, and thrombotic events (11%). In addition, she said that some patients reported feeling "off balance," which may have been more related to the thalidomide.
Because toxicity was dose-related and response in the M.D. Anderson studies did not improve at doses greater than 400 mg, the investigators suggest that future studies focus on thalidomide doses of 400 mg or less. They also call for further study of the thalidomide/dexamethasone combination as a substitute for intensive therapy in relapsing disease and for those with previously untreated disease. Dr. Weber cited M.D. Anderson results showing that thalidomide alone, while active, produced response rates of only 35% in patients with early asymptomatic disease.
Newly Diagnosed Disease
A Mayo Clinic study found that the combination of thalidomide and dexamethasone produced response rates of 77% in patients with newly diagnosed multiple myeloma. "This is the first study that shows the benefit of this novel combination of drugs for patients with previously untreated myeloma," reported S. Vincent Rajkumar, MD, the study’s lead author.
The combination of these two drugs may represent an entirely oral alternative to intravenous chemotherapy, namely VAD, he said.
Thalidomide was administered at 200 mg/d. Although the initial protocol called for dose escalation up to 800 mg/d, the protocol was amended after grade 3-4 skin toxicity occurred in 2 of the first 7 patients. For the next 19 patients, the thalidomide dose was kept constant at 200 mg/d.
The dexamethasone schedule was 40 mg/d orally on days 1 to 4, 9 to 12, and 17 to 20 on odd cycles, and on days 1 to 4 on even cycles, repeated monthly.
A response, defined as a decrease in serum and urine myeloma protein by 50% or greater, was confirmed in 20 patients (77%). Response was higher (84%) among patients whose thalidomide dose was escalated. The response rate was 74% among those whose thalidomide dose remained constant at 200 mg/d.
The study also included 16 patients with smoldering or indolent multiple myeloma, Dr. Rajkumar told ONI. These patients were treated with thalidomide alone. The confirmed response rate was 38% (6 of 16 patients). If minor re-sponses (25% to 49% reduction in myeloma protein) are included, however, the response rate rises to 69%.
Toxicities included skin rash, sedation, constipation, and neuropathy.
Targeted Therapy First Strategy Reduces Need for Chemotherapy in Newly Diagnosed LBCL
December 7th 2025Lenalidomide, tafasitamab, rituximab, and acalabrutinib alone may allow 57% of patients with newly diagnosed LBCL to receive less than the standard number of chemotherapy cycles without compromising curative potential.