The Value of Pelvic Radiation Therapy After Hysterectomy for Early Endometrial Cancer
In this review, the results and limitations of studies concerning adjuvant radiation therapy and chemotherapy for endometrial cancer will be discussed, focusing on evidence that can help to guide treatment decisions.
Figure 1: Nomogram Proposed by Alhilli et al for the Prediction of Lymph Node Metastases
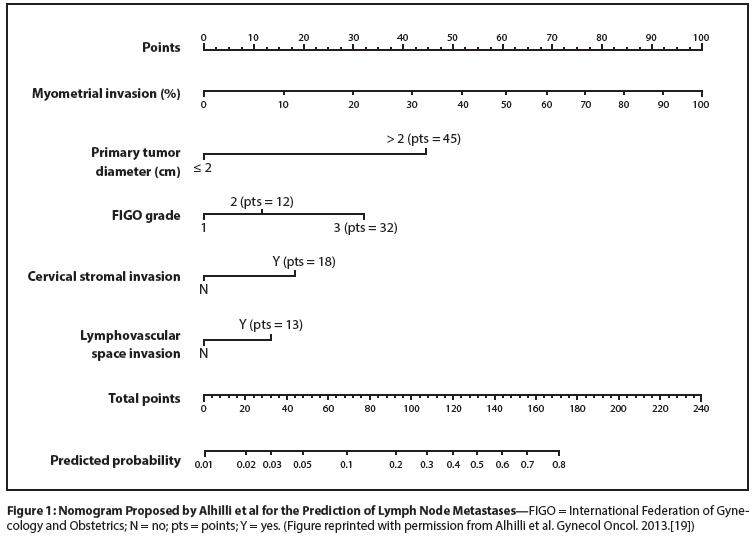
Figure 2: Overall Survival of 265 Patients With Optimally Resected Stage IIIC Endometrial Cancer
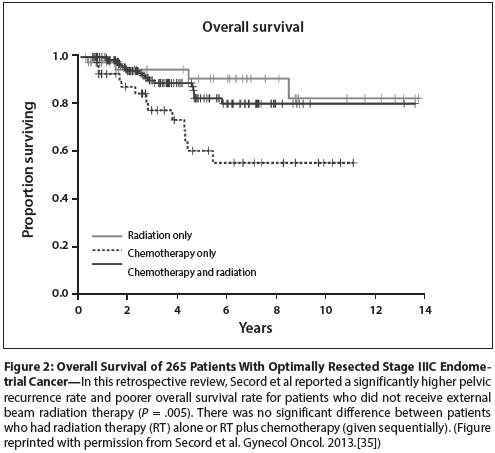
Figure 3: Overall Survival of Patients Treated in the Gynecologic Oncology Group 99 Trial
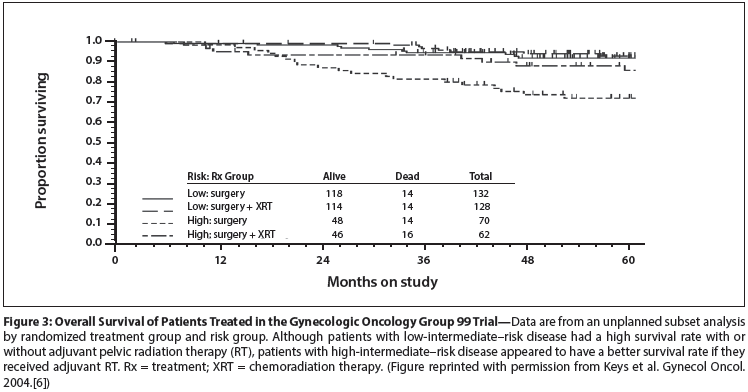
Figure 4: Progression-Free (PF) Survival (Gray) and Overall Survival (Black) for Patients Treated With Either Whole-Abdominal Radiation Therapy (WAI) or Chemotherapy (Doxorubicin and Cisplatin [AP]) in the Gynecologic Oncology Group 122 Trial
Table: Randomized Trials Evaluating the Roles of Adjuvant RT in Patients With Endometrial Cancer (EC)
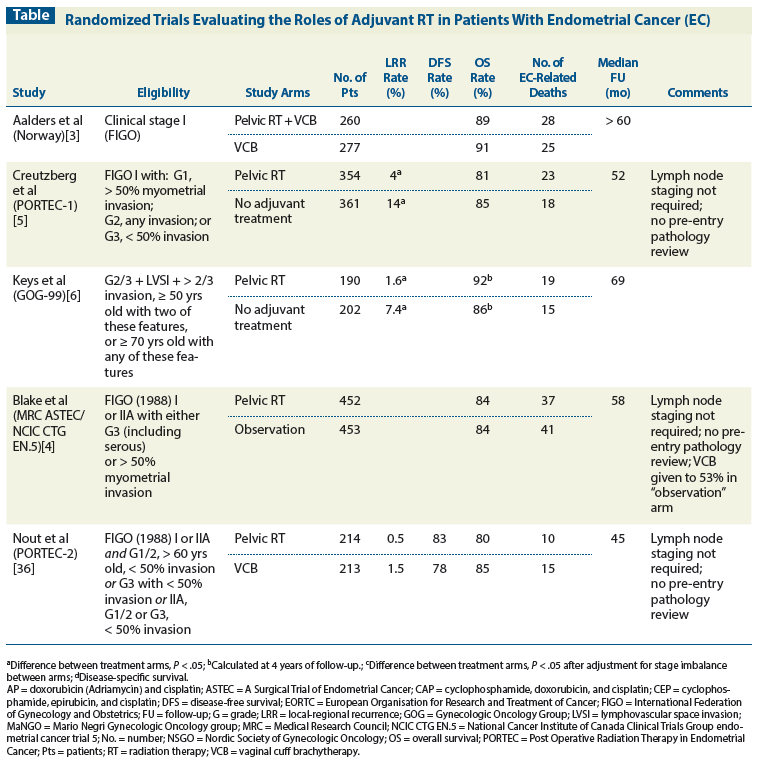
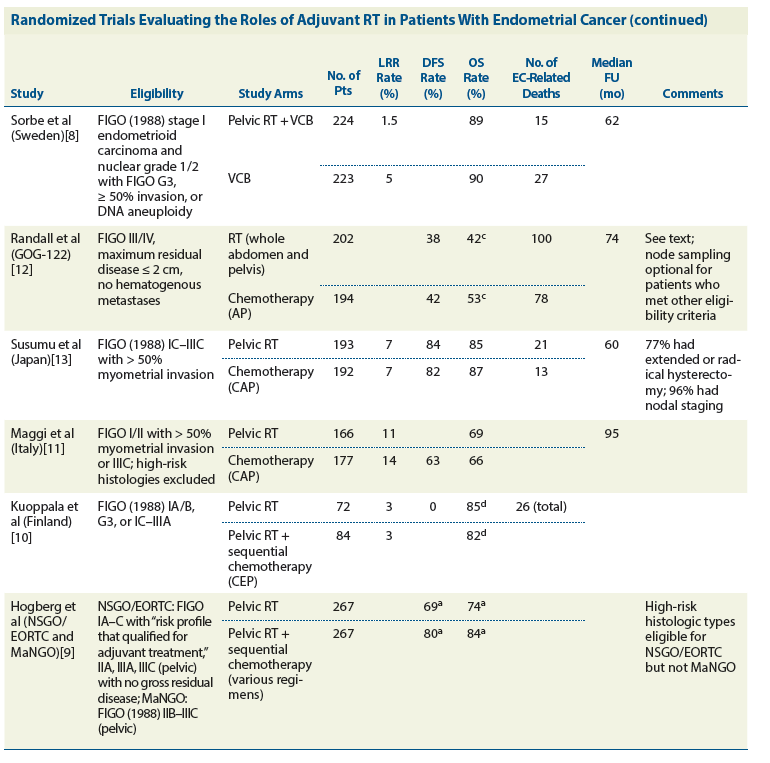
Table (continued): Randomized Trials Evaluating the Roles of Adjuvant RT in Patients With Endometrial Cancer (EC)
Although endometrial carcinoma is one of the most common cancers affecting women, most cases are detected at an early stage and are cured with hysterectomy alone. Most recurrences occur in the relatively small subset of patients whose surgical specimens reveal multiple risk factors. Clinicians have sought to define adjuvant treatments that can improve the outcome of treatment for these higher-risk patients. Although randomized trials have demonstrated that radiation therapy improves local control, they have failed to demonstrate an improvement in survival with radiation therapy. In this review, the results and limitations of studies concerning adjuvant radiation therapy and chemotherapy for endometrial cancer will be discussed, focusing on evidence that can help to guide treatment decisions.
Introduction
Endometrial cancer is the most common gynecologic malignancy diagnosed in the United States. In 2013, an estimated 49,560 new cases of endometrial cancer were diagnosed, and there were 8,190 deaths from this disease.[1] Decreases in incidence rates that paralleled changing patterns of hormone replacement therapy in the late 20th century appear to have reversed in recent years as the rate of obesity, which is associated with increased levels of endogenous estrogens, has increased.[2] Endometrial cancer is an important public health problem that affects hundreds of thousands of women worldwide; thus, changes in the established standards for treatment can have major implications at the societal level for the health and welfare of women, and for the cost of their medical care.
Most endometrial cancers are diagnosed at an early stage, after a woman notices abnormal, usually postmenopausal, bleeding. Because most endometrial cancers also have relatively favorable biologic characteristics, hysterectomy alone usually is sufficient to cure the disease. Although a small proportion of patients have distant metastases detected at initial diagnosis, most of the deaths from endometrial cancer occur in patients who initially appear to have locoregionally confined disease. To the extent that their risk of recurrence can be accurately predicted at the time of diagnosis, these are the patients who stand to benefit from effective adjuvant treatments.
For decades, researchers have worked to perfect various forms of adjuvant treatment and determine their effectiveness for endometrial cancer. We can now refer to the results of numerous prospective randomized trials that have tested the benefits of adjuvant radiation therapy (RT),[3-8] chemotherapy,[9-13] and lymphadenectomy in this setting.[14,15] Unfortunately, critical review of these trials does not yield convincing evidence of benefit, at least in terms of overall survival, for any treatment beyond hysterectomy. Yet all of these adjuvant treatments continue to have passionate advocates, and the controversies surrounding treatment of endometrial cancer sometimes appear to be no more conclusively settled than they were before trials of various regimens were concluded. This situation is reflected in the current guidelines of the National Comprehensive Cancer Network, which continue to include a wide variety of treatment options for most subgroups of patients with locoregionally confined disease; none of these options are supported by high-level evidence.[16]
An important-possibly the most important-reason that these trials have failed to yield definitive conclusions is that their eligibility criteria often did not define a population that stood to gain enough from adjuvant treatment for a benefit to be detectable in the overall analysis. Many trials included large numbers of patients who had minimal risk of recurrence, and some included patients whose disease was so extensive that they could not benefit from locoregional treatment. Both of these factors decreased the power of certain trials to detect or rule out benefit to more appropriately selected patients.
Nevertheless, we have made progress over the past 30 years. We have gained confidence in the safety of withholding all adjuvant treatment for patients in low-risk categories. We have gained information about the biologic characteristics of endometrial cancer, its natural history, and risk factors for development of recurrent endometrial cancer. We have learned more about the side effects that must be considered in balancing risks against potential benefits of treatment for individual patients. We also have some hints of real benefit from adjuvant RT and possibly chemotherapy for some patients who have cancers with high-risk features.
Who Stands to Benefit From Effective Adjuvant Treatment?
To obtain a meaningful answer to the question of which patients with endometrial cancer stand to benefit from effective adjuvant treatment, one must first ask and answer at least three related questions. First, who is at risk for developing recurrence after hysterectomy alone? Second, what is the likely pattern of recurrence, and will the proposed form of adjuvant treatment adequately address it? Third, what is the likelihood of successful treatment of a recurrence with available therapies if no adjuvant treatment is given? Ultimately, to determine the net therapeutic benefit, one must ask two additional questions: what are the side effects of adjuvant treatment, and how do they compare with the consequences of no adjuvant treatment? Even a potentially effective adjuvant treatment will fail as a therapeutic strategy if these questions have not been adequately considered and answered.
Who is at risk of developing recurrence?
Several morphologic and microscopic characteristics of disease within the hysterectomy specimen, including histologic grade and subtype, tumor size, myometrial invasion, cervical stromal invasion, and lymphovascular space invasion (LVSI), are correlated with the probabilities of extrauterine disease and disease recurrence.[17-19]
Most instances of extrauterine disease or disease recurrence occur in the minority of patients whose tumors exhibit several risk factors. For example, using a database of 883 patients with endometrioid endometrial cancers, Alhilli et al[19] recently created a nomogram for risk of lymph node involvement (Figure 1). According to their nomogram, for a patient with an International Federation of Gynecology and Obstetrics (FIGO) grade 3 tumor larger than 2 cm, if the patient had 70% myometrial invasion and LVSI, the predicted risk of lymph node involvement would be more than 40%. In contrast, if the patient had 70% myometrial invasion but no other high-risk features, or minimal myometrial invasion and only one of the other high-risk features, the predicted risk would be less than 5%. Although this nomogram has not yet been validated, the complex interactions between risk factors for patients with endometrial cancer suggest that a similar method would be a more effective guide for treatment selection than the more simplistic methods currently in general use.
The FIGO staging system for endometrial cancer incorporates tumor grade, myometrial invasion, and cervical stromal invasion in its classification, using a threshold of 50% myometrial invasion for the distinction between stages Ia and Ib. Although there is some virtue in simplicity, dichotomization of this variable undoubtedly reduces its predictive power. Various authors have argued for using different thresholds,[6] for using absolute rather than fractional measurements of invasiveness,[20] or for treating invasion as a continuous variable in a nomogram like that proposed by Alhilli et al.[19] Although the results depend somewhat on the composition of the study population, these authors have all made strong arguments for their alternative methods of classifying myometrial invasion.
Tumor size, which is not included in the FIGO staging system, has also been reported to be an independent predictor of risk and appears to be particularly strongly correlated with the risk of lymph node involvement.[19,21]
High tumor grade and the presence of high-risk histologic subtypes, particularly serous carcinoma, are also associated with poor outcome.
LVSI has repeatedly been demonstrated to be an independent predictor of lymph node involvement and recurrence; the degree of risk may be further associated with the extent of LVSI in the uterine specimen.[22]
For patients who undergo lymphadenectomy at the time of hysterectomy, the finding of lymph node metastasis has been associated with a high risk of recurrence.[23] The number of positive nodes and the ratio of positive lymph nodes to the total number of nodes have also been correlated with outcome.[24] However, most patients with positive lymph nodes do receive some form of adjuvant treatment, and their risk may also depend on the type of therapy they receive.
Peritoneal cytology is now known to be a poor independent predictor of outcome in patients with endometrial cancer, and this feature has been removed from the staging system. The finding of malignant cells in peritoneal fluid acted in part as a surrogate marker for high-risk histologic subtypes, but the presence of malignant cells in peritoneal fluid does not necessarily mean that those cells can implant and grow on peritoneal surfaces.
The other major factor that has been strongly associated with recurrence of apparently limited endometrial cancers is older age. Although high-risk histologic subtypes tend to occur in older women, the predictive power of age appears to be independent of histologic subtype, grade, and other known correlates of outcome. Although older age is likely a surrogate variable for some as-yet-unidentified biologic causes of aggressive tumor behavior, it is currently one of the factors used to identify patients who might benefit from adjuvant treatment.
What is the likely pattern of recurrence?
Staging studies suggest that about 15% of women with endometrial cancer have lymph node metastases at the time of diagnosis.[25] Endometrial cancer can spread to the external or internal iliac nodes or directly to the aortic nodes; the most likely site of direct aortic node spread is to the nodes above the inferior mesenteric artery. Estimates of the proportion of patients with lymph node metastases having para-aortic involvement range from 30% to 67%, although recurrence patterns suggest that the real level of para-aortic involvement may be somewhere in between these figures.[25,26] About 10% to 15% of patients with node-positive disease have only para-aortic metastases.
These patterns of disease spread have important implications for adjuvant treatment. One argument against adjuvant pelvic RT in high-risk patients is that these patients also have a high risk of para-aortic disease that would not be covered by standard pelvic fields. All of the intrauterine risk factors described above have been demonstrated repeatedly to be independent predictors of lymph node involvement. However, lymphatic vessels generally do not reach the endometrium, and even high-grade tumors rarely have evidence of nodal involvement unless there is significant myometrial or cervical stromal involvement.
The most common site of endometrial cancer recurrence is the vagina, and most vaginal recurrences are located in the vaginal apex. The proportion of patients at risk for vaginal recurrence is difficult to estimate because in most series the higher-risk patients undergo adjuvant treatment; however, for patients who receive no adjuvant treatment for high-risk disease, the vaginal recurrence rate may be 20% to 30% or more.
It is often assumed that vaginal recurrences are a result of lymphovascular invasion. However, there are several arguments against this theory. Most compelling is the fact that vaginal recurrences usually can be prevented with a modest dose of superficial vaginal brachytherapy. It is difficult to imagine an anatomic explanation for a pattern of tumor spread from the uterine fundus that involves lymphatic vessels of the vagina but not those of other paracervical tissues. Although vaginal metastases are occasionally present at the time of initial diagnosis, this situation is very rare, occurring in less than 1% of patients with newly diagnosed endometrial cancer. Another argument against the theory of lymphovascular spread as a mechanism for vaginal recurrence is the fact that vaginal recurrences are seen with some frequency in patients with minimally invasive, even noninvasive, cancers, particularly high-grade cancers. In the Postoperative Radiation Therapy in Endometrial Carcinoma (PORTEC)-1 trial,[5] the vaginal recurrence rate was 18% for patients who had stage IA, grade 3 disease; however, approximately half of the patients with these intrauterine features received pelvic RT, and the vaginal recurrence rate was likely much higher for the patients who received no adjuvant RT. These observations, combined with the fact that most vaginal recurrences are in the region of the apical vaginal incision, suggest that scar implantation is a more likely mechanism than lymphatic metastasis for most vaginal recurrences. The distinction between these two mechanisms has important implications for adjuvant treatment: if the mechanism of recurrence is primarily scar implantation, this would help to distinguish patients at risk primarily for vaginal recurrence from those who might benefit from more comprehensive regional treatment.
Histologic subtype also provides important clues about the likely pattern of disease recurrence. Intraperitoneal dissemination is very rare in patients who have endometrioid cancers, but it is common in those with serous subtypes, even when there is no evidence of myometrial invasion.[27,28] Pelvic RT alone is probably not appropriate for patients whose recurrence risk is primarily intraperitoneal, although vaginal recurrence is also a risk in such patients.
What is the likelihood of successful treatment of a recurrence?
Although most vaginal recurrences can clearly be prevented with adjuvant RT, the impact of these successes on survival is diminished because many vaginal recurrences are isolated and can be successfully treated with RT after they occur. Although patients who received no adjuvant treatment in the PORTEC-1 trial had an overall probability of vaginal recurrence of 10%, many of these recurrences were successfully treated, contributing to the lack of an overall survival benefit with adjuvant pelvic RT.[5] However, most of the patients in this trial had grade 1 disease, and most of the vaginal recurrences occurred in patients with grade 1 disease. Jhingran et al have demonstrated that the likelihood of successful treatment of vaginal recurrence is strongly correlated with tumor grade[29]: although grade 1 recurrences can usually be treated successfully, in their review, only about 40% of patients with apparently isolated high-grade vaginal recurrences were cured with pelvic RT and brachytherapy.
Fewer data are available regarding rates of successful treatment of extravaginal local recurrences. In the past, it was difficult to safely deliver a sufficient radiation dose to sterilize gross recurrent disease in pelvic or aortic nodes. Modern intensity-modulated RT techniques have made it safer to deliver tumoricidal doses, and this has encouraged clinicians to attempt treatment of such recurrences with RT. In a review of patients with aortic node recurrences of endometrial cancer, Shirvani et al reported a 53% disease-free survival rate at 3 years.[30]
For patients with recurrent or metastatic disease, rates of response to multiagent chemotherapy are as high as 50% to 60%. However, cures with chemotherapy alone are rare. Five-year survival rates are less than 10% in most studies.[31]
What are the risks of adjuvant RT?
All treatments for endometrial cancer have side effects. Although substantial side effects may be tolerated if they are necessary to achieve cure, even minor treatment-related toxic effects are unacceptable if the treatment has no benefit.
Nout et al reported that patients who had pelvic RT for endometrial cancer were more likely to voice long-term complaints of chronic diarrhea, rectal urgency, and fecal incontinence than were patients treated with surgery or vaginal brachytherapy alone.[32] Bowel obstructions are uncommon, but they are more frequently seen in patients treated with adjuvant RT than in those who do not receive such treatment. The risk of complications is greater in very thin women and in smokers. Modern conformal techniques such as intensity-modulated RT decrease the volume of tissue irradiated and the severity of acute side effects, and they may decrease the risk of long-term bowel complications. Vaginal brachytherapy is associated with fewer side effects than pelvic RT, but it can cause vaginal shortening or narrowing. The extent of vaginal injury and the likelihood of other side effects are related to the treatment schedule and the length of vagina irradiated.[33]
Lymphadenectomy has been advocated as a way of triaging patients to reduce the number of patients referred for adjuvant RT. However, lymphadenectomy is also associated with short-term and long-term side effects-including lymphedema, lymphocyst formation, and wound complications-and with increased risk of radiation side effects if adjuvant RT is required. Sentinel lymph node evaluation is currently being explored as a possible way to identify high-risk patients without the side effects of comprehensive lymphadenectomy.
Does RT Contribute to Cure in Patients With Endometrial Cancer?
There are now at least six prospective randomized trials that have compared adjuvant pelvic RT with either no adjuvant treatment or postoperative vaginal brachytherapy only (Table).[3-8] All of these studies have demonstrated improved pelvic disease control rates with pelvic RT, but none have demonstrated a significant improvement in overall survival with pelvic RT.
On the basis of these results, some clinicians have concluded that there is no role for adjuvant external beam irradiation; perhaps a few have even argued that there is no role for RT at all in the treatment of endometrial cancer. However, there is little doubt that endometrial cancer can be cured with RT. Respectable long-term survival rates have been reported after RT for patients with medically inoperable disease; for patients with vaginal, pelvic, or aortic recurrences; and for patients with stage IIIC disease. Undoubtedly many such patients who would otherwise have died of their disease were cured with RT.
The outcomes of patients with stage IIIC disease are of particular interest. Several studies have suggested that regional RT is highly effective in patients who have known lymph node metastases. In a retrospective study of 71 patients with stage IIIC disease,[34] the pelvic disease control rate was 98% for patients treated with external beam irradiation but only 61% for patients treated with only systemic therapy with or without vaginal cuff irradiation. More importantly, the 5-year overall survival rate also was significantly better for patients who had external beam therapy (70% vs 43%). Notably, several of the long-term survivors who were treated with initial systemic therapy had recurrence after chemotherapy but were subsequently successfully treated with definitive RT. In a more recent multicenter retrospective review, by Secord et al,[35] patients treated for stage IIIC disease with either radiation only or radiation plus chemotherapy had significantly better local control, relapse-free survival, and overall survival rates than patients treated with chemotherapy only (Figure 2); this was true despite the fact that patients treated with radiation only or radiation plus chemotherapy were much more likely to have grade 3 cancers than were patients treated with chemotherapy only. In this series, patients treated with chemotherapy only were significantly more likely to experience pelvic disease recurrence than were those who had RT. The authors do not state how many of the chemotherapy-only survivors had isolated locoregional recurrences after chemotherapy that were successfully treated with RT.
If regional treatment is so effective in these high-risk cases, why was it not more effective in the randomized trials? If RT can cure known regional metastases, why would it not be curative for patients at risk for locoregional recurrence? Certainly in other types of cancer-for example, head and neck cancer and cervical cancer-RT has been effective in both types of patients.
The answer undoubtedly lies in the eligibility criteria of the trials of adjuvant RT for endometrial cancer (Table).[3-8] At least in retrospect, it is clear that the profiles of patients entered in these trials were much too favorable for any study of adjuvant treatment; the total number of cancer-related events was small, and the overall survival rates of untreated patients in the control arms were in all cases greater than 80% to 85%. Once central pathology review was performed on patients entered in the PORTEC-1 and PORTEC-2 trials, it became apparent that community facilities had routinely over-graded the lesions. In the end, most of the patients entered in these trials had grade 1 disease; the accuracy of initial readings of myometrial penetration is unknown. Two-thirds of the patients entered in the Gynecologic Oncology Group 99 (GOG-99) trial had what would now be termed “low-intermediate–risk” disease; these patients had an overall survival rate without treatment of more than 90%. Certainly one can conclude from these trials that for patients with minimally invasive grade 1 or 2 cancers and many more deeply invasive grade 1 cancers, the benefits of pelvic RT are negligible and outweighed by the costs in terms of side effects and resources.
On the other hand, the one trial that included both surgical staging and expert pathology review, GOG-99 (Figure 3), did suggest that pelvic RT may improve survival if sufficiently high-risk patients are selected for study.[6] In a subset analysis of 132 patients deemed to have high-intermediate–risk disease (using a schema that incorporated age, depth of invasion, and tumor grade), the authors found that patients who received adjuvant RT had an overall survival rate that was approximately 20% greater than that of patients who received no adjuvant treatment. Because many of the recurrences in the control arm were in the vaginal apex, the authors speculated that a similar benefit might be achieved with vaginal cuff irradiation only. PORTEC-2 was designed to answer this question. The results of PORTEC-2 demonstrated a similar survival rate for patients who had pelvic RT and those who had vaginal cuff RT; however, the patients entered in PORTEC-2 had substantially more favorable lesions than those defined by the GOG high-intermediate designation. For this reason, many clinicians continue to consider adjuvant pelvic RT to be standard for the GOG-defined high-intermediate–risk group.
Is Chemotherapy More Effective Adjuvant Treatment Than RT?
Although clinicians are increasingly using chemotherapy as an adjuvant treatment for patients with high-risk or high-intermediate–risk endometrial cancer, there is as yet no solid evidence that chemotherapy improves overall survival. Two randomized trials comparing adjuvant pelvic RT vs the combination of cisplatin, doxorubicin, and cyclophosphamide revealed no difference in overall survival between the arms.[11,13]
The trial that has most often been quoted in support of chemotherapy, GOG-122,[12] compared the combination of cisplatin and doxorubicin vs whole-abdominal RT in patients with high-risk disease (stage III and IV confined to the abdomen and pelvis). Although the relapse-free survival rates were similar in the two groups, patients who received chemotherapy had significantly better overall survival than those treated with whole-abdominal RT. Interestingly, patients who received chemotherapy had a 5-year overall survival rate that was substantially higher than their relapse-free survival rate, suggesting that salvage treatment contributed importantly to their overall survival (Figure 4). In their report of the trial, the authors stated that patients who received chemotherapy were much more likely than patients who received whole-abdominal RT to receive RT as treatment for relapsed disease. Unfortunately, the dose and schedule of whole-abdominal RT prescribed in this 1992 trial were, at least in retrospect, inadequate to control the burden of cancer in these high-risk patients, many of whom were known to have gross residual disease. The prescribed whole-abdominal RT was, however, sufficient to preclude any chance of delivering definitive RT for subsequent recurrences. It is ironic that the better survival rate of patients treated with chemotherapy in this trial may well be due, at least in part, to the fact that their initial treatment did not preclude definitive, targeted RT for their subsequent recurrences.
Interestingly, the most compelling evidence for a benefit from chemotherapy may be the results of a recent multi-institutional European trial published by Hogberg et al.[9] The trial, which compared adjuvant pelvic RT with or without various combinations of sequential chemotherapy, demonstrated significantly better progression-free survival for patients who received chemotherapy (P = .009), although the difference in overall survival was not statistically significant. While studies of combined-modality treatment have produced mixed results,[9,35] optimization of combined-modality treatment may be a fruitful avenue for future study in patients with particularly high-risk disease.
Conclusions
In summary, most recurrences in patients with endometrial cancer occur in patients who have multiple high-risk features. Minimally invasive, grade 1 or 2 disease without other high-risk features rarely, if ever, requires any form of adjuvant treatment. Grade 3 cancers are associated with a high risk of vaginal recurrence and probably warrant treatment with at least vaginal cuff irradiation. In patients with high-risk and high-intermediate–risk endometrioid cancer, adjuvant external beam RT significantly reduces the rate of local recurrence. For patients who have stage I or II disease with multiple high-risk features, pelvic RT continues to be a standard treatment. However, the benefit of this treatment has not yet been proven in well-designed, adequately powered trials. For patients with stage IIIC (node-positive) endometrioid cancer, RT is an effective treatment that yields high survival rates, particularly for those women with grade 1 or 2 disease. The benefit of adjuvant chemotherapy for endometrial cancer has not yet been clearly demonstrated, although results from studies of combined-modality treatment are encouraging.
Disclosures:
The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30.
2. McCullough ML, Patel AV, Patel R, et al. Body mass and endometrial cancer risk by hormone replacement therapy and cancer subtype. Cancer Epidemiol Biomarkers Prev. 2008;17:73-9.
3. Aalders J, Abeler V, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980;56:419-27.
4. Blake P, Swart AM, Orton J, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137-46.
5. Creutzberg CL, van Putten WL, Warlam-Rodenhuis CC, et al. Outcome of high-risk stage IC, grade 3, compared with stage I endometrial carcinoma patients: the Postoperative Radiation Therapy in Endometrial Carcinoma Trial. J Clin Oncol. 2004;22:1234-41.
6. Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744-51.
7. Nout RA, Smit VT, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816-23.
8. Sorbe B, Horvath G, Andersson H, et al. External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium-risk endometrial carcinoma-a prospective randomized study. Int J Radiat Oncol Biol Phys. 2012;82:1249-55.
9. Hogberg T, Signorelli M, de Oliveira CF, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer-results from two randomised studies. Eur J Cancer. 2010;46:2422-31.
10. Kuoppala T, Maenpaa J, Tomas E, et al. Surgically staged high-risk endometrial cancer: randomized study of adjuvant radiotherapy alone vs. sequential chemo-radiotherapy. Gynecol Oncol. 2008;110:190-5.
11. Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266-71.
12. Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36-44.
13. Susumu N, Sagae S, Udagawa Y, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-33.
14. Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707-16.
15. Kitchener H, Swart AM, Qian Q, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125-36.
16. National Comprehensive Cancer Center Network. Uterine neoplasms. 2013; Available from: http://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
17. Zaino RJ, Kurman RJ, Diana KL, Morrow CP. Pathologic models to predict outcome for women with endometrial adenocarcinoma: the importance of the distinction between surgical stage and clinical stage--a Gynecologic Oncology Group study. Cancer. 1996;77:1115-21.
18. Creasman W, Morrow C, Bundy B, et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60:2035-41.
19. Alhilli MM, Podratz KC, Dowdy SC, et al. Risk-scoring system for the individualized prediction of lymphatic dissemination in patients with endometrioid endometrial cancer. Gynecol Oncol. 2013 Jul 9. [Epub ahead of print]
20. Geels YP, Pijnenborg JM, van den Berg-van Erp SH, et al. Absolute depth of myometrial invasion in endometrial cancer is superior to the currently used cut-off value of 50%. Gynecol Oncol. 2013;129:285-91.
21. Schink JC, Rademaker AW, Miller DS, Lurain JR. Tumor size in endometrial cancer. Cancer. 1991;67:2791-4.
22. Hachisuga T, Kaku T, Fukuda K, et al. The grading of lymphovascular space invasion in endometrial carcinoma. Cancer. 1999;86:2090-7.
23. Morrow C, Bundy B, Kurman R, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55-65.
24. Polterauer S, Khalil S, Zivanovic O, et al. Prognostic value of lymph node ratio and clinicopathologic parameters in patients diagnosed with stage IIIC endometrial cancer. Obstet Gynecol. 2012;119:1210-8.
25. Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11-8.
26. Morrow CP, Bundy BN, Kurman RJ, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55-65.
27. Hendrickson M, Ross J, Eifel P, et al. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6:93-108.
28. Silva EG, Jenkins R. Serous carcinoma in endometrial polyps. Modern Pathol. 1990;3:120-8.
29. Jhingran A, Burke TW, Eifel PJ. Definitive radiotherapy for patients with isolated vaginal recurrence of endometrial carcinoma after hysterectomy. Int J Radiat Oncol Biol Phys. 2003;56:1366-72.
30. Shirvani SM, Klopp AH, Likhacheva A, et al. Intensity modulated radiation therapy for definitive treatment of paraortic relapse in patients with endometrial cancer. Pract Radiat Oncol. 2013;3:e21-e28.
31. Fleming GF, Filiaci VL, Bentley RC, et al. Phase III randomized trial of doxorubicin + cisplatin versus doxorubicin + 24-h paclitaxel + filgrastim in endometrial carcinoma: a Gynecologic Oncology Group study. Ann Oncol. 2004;15:1173-8.
32. Nout RA, Putter H, Jurgenliemk-Schulz IM, et al. Five-year quality of life of endometrial cancer patients treated in the randomised Post Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial and comparison with norm data. Eur J Cancer. 2012;48:1638-48.
33. Sorbe B, Straumits A, Karlsson L. Intravaginal high-dose-rate brachytherapy for stage I endometrial cancer: a randomized study of two dose-per-fraction levels. Int J Radiat Oncol Biol Phys. 2005;62:1385-9.
34. Klopp AH, Jhingran A, Ramondetta L, et al. Node-positive adenocarcinoma of the endometrium: outcome and patterns of recurrence with and without external beam irradiation. Gynecol Oncol. 2009;115:6-11.
35. Secord AA, Geller MA, Broadwater G, et al. A multicenter evaluation of adjuvant therapy in women with optimally resected stage IIIC endometrial cancer. Gynecol Oncol. 2013;128:65-70.
36. Nout RA, Putter H, Jürgenliemk-Schulz IM, et al. Vaginal brachytherapy versus external beam pelvic radiotherapy for high-intermediate risk endometrial cancer: Results of the randomized PORTEC-2 trial. J Clin Oncol. 2008;26(suppl 15S):Abstr LBA5503.