A Watch-and-Wait Approach to the Management of Rectal Cancer
This article reviews current evidence in support of a watch-and-wait approach to rectal cancer management, and discusses the challenges and limitations of this approach.
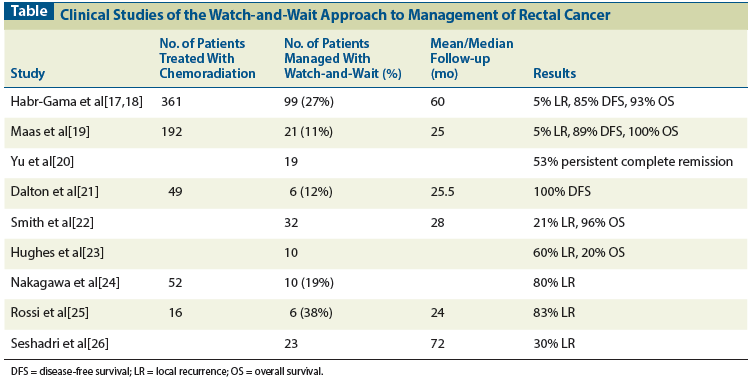
Table: Clinical Studies of the Watch-and-Wait Approach to Management of Rectal Cancer
There has been increasing interest in whether a watch-and-wait strategy can be pursued instead of routine surgery in selected rectal cancer patients who have a clinical complete response (cCR) after chemoradiation. The watch-and-wait approach could potentially reduce treatment-related toxicity in selected rectal cancer patients. A large study from Brazil and a prospective trial from the Netherlands appear to support this approach, although multiple other studies have raised concerns about the high rate of local recurrence with this strategy. This article reviews current evidence in support of a watch-and-wait approach to rectal cancer management, and discusses the challenges and limitations of this approach. Among these are the facts that current methods of assessing tumor response have limited accuracy, and that a cCR does not necessarily imply pathologic complete response. Careful patient selection and systematic methods of response assessment and follow-up will be critical to the success of nonoperative approaches. Based on the available evidence, ideally a watch-and-wait approach for patients with rectal cancer should be pursued within the context of a prospective clinical trial.
Introduction
During the last few decades, treatment intensification has been the dominant paradigm for the management of rectal cancer. Surgery has evolved from less radical approaches to total mesorectal excision (TME).[1] Moreover, combined-modality therapy has become widely accepted as the standard for all but the earliest stages of resectable rectal cancer. Most patients with stage II and III rectal cancer are now treated with preoperative chemoradiation or preoperative short-course radiotherapy, prior to TME.[2,3] In addition, postoperative or adjuvant chemotherapy is commonly used for patients with stage II and III rectal cancer.[4]
In contrast to treatment intensification with more radical surgery and combined-modality therapy, treatment de-escalation is also an area of increasing interest in rectal cancer. Treatment de-escalation could potentially reduce treatment-related toxicity without compromising oncologic outcomes in selected rectal cancer patients. Treatment de-escalation also may offer the potential for use of less radical or less intense treatments, or even for the omission of specific treatment modalities. For instance, some studies have evaluated local excision instead of TME, while others have evaluated omission of preoperative radiotherapy in selected patients.[5-8]
An intriguing question that has emerged is whether a watch-and-wait approach could be pursued for selected rectal cancer patients after chemoradiation, as an alternative to routine surgery. Preoperative chemoradiation yields pathologic complete response (pCR) rates of about 10% to 20% in rectal cancer patients.[9-13] Hence, a subset of rectal cancer patients could potentially not require surgery after chemoradiation. While it is difficult to accurately determine which patients will have a pCR to chemoradiation, some studies have evaluated a watch-and-wait approach in patients with a clinical complete response (cCR) to chemoradiation. We will review the current evidence for a watch-and-wait approach toward rectal cancer, and discuss the challenges and limitations of this approach.
Radiotherapy Without Surgery: Long-Term Results
A small number of studies have evaluated long-term outcomes in patients treated by radiation therapy without surgery for rectal cancer. Investigators from the Princess Margaret Hospital reported a series of 271 patients treated with radiation therapy without surgery, between 1978 and 1987.[14] Most patients were treated in the earlier years, with only 19 patients treated in the last 5 years of the study. The series included patients who were medically inoperable or surgically unresectable, or who refused surgery. A variety of radiation techniques and dose regimens were used; the minimum dose was 40 Gy, and the median and most common dose was 52 Gy in 20 fractions. No patient received concurrent chemotherapy. Seventy-eight patients subsequently underwent surgery for persistent or recurrent disease. The tumor response and survival rates varied depending on whether patients had mobile, partially fixed, or fixed tumors. The cCR rates were 49%, 22%, and 9%, and the 5-year overall survival (OS) rates were 48%, 26%, and 6%, for patients with mobile, partially fixed, and fixed tumors, respectively. Among patients who had an initial cCR, 78% subsequently developed local relapse. Thus, this series showed reasonable cCR and survival rates with radiation alone, although the rates of local relapse were very high. However, it is difficult to make generalizations from this study because of the heterogeneity of patient characteristics and radiation doses used.
A study in Lyon, France, evaluated high-dose radiation therapy without surgery for rectal cancer in 63 patients with T2-T3 N0-N1 rectal cancer, treated between 1986 and 1998.[15] Patients were treated with a three-step course of radiotherapy: contact x-rays (80 Gy in 3 fractions), followed by external beam radiation (39 Gy in 13 fractions with a concomitant boost of 4 Gy in 4 fractions), followed by low-dose-rate brachytherapy with an iridium implant (20 Gy). A total of 92% of patients had clinical complete response. Eight patients subsequently underwent abdominoperineal resection (APR) for residual or recurrent disease. With a median follow-up of 54 months, the ultimate pelvic control rate was 73%, and the 5-year OS rate was 64%. Most patients (65%) in this study had T2 disease, and these patients had a higher OS rate compared with the patients with T3 disease (84% vs 53%). The high-dose radiation regimen did result in late toxicity in a significant proportion of patients, with 38% developing rectal bleeding, typically lasting 2 to 3 years, and 19% developing grade 2 rectal necrosis. The three-step radiation regimen used in this study requires technical expertise and experience, and it may be difficult to replicate at other centers. The lower survival rate in T3 patients and the relatively high rates of late toxicity seen in this study are also of concern. However, this study did provide proof of principle that radiation therapy alone can lead to acceptable local control and survival rates in selected rectal cancer patients.
A more recent retrospective study from six Australian institutions evaluated 48 patients treated for rectal cancer with radiation without surgery, between 1998 and 2005.[16] Half of the patients in this series refused surgery, and the other half were inoperable for medical reasons. The majority of patients (66%) had T3 disease, while the rest had T1-T2 disease. The median radiation dose was 50 Gy in 25 fractions, and 75% of the patients received concurrent chemotherapy. A total of 56% of patients had a cCR. With a median follow-up of 49 months, 38% of patients had disease progression. The median progression-free survival (PFS) was 65 months in patients with a cCR, and 15 months in patients with a partial response. There were 25 deaths; 16 were due to rectal cancer and 9 were due to other causes. Thus, this study indicates that conventional doses of radiation with concurrent chemotherapy can achieve high rates of disease control without surgery in rectal cancer patients.
The studies discussed above have been based on heterogeneous patient populations, and they have included both clinical complete responders and partial responders. We will next review studies that have selected patients based on response and have evaluated non-surgical approaches only among patients achieving a cCR.
Radiotherapy Without Surgery in Patients With Clinical Complete Response
Investigators from Brazil (Habr-Gama et al) conducted a systematic study of a non-operative approach for clinical complete responders.[17,18] In an updated analysis, these investigators reported on 361 patients with distal rectal adenocarcinoma who were treated with radiation therapy at a dose of 50.4 Gy, along with concurrent 5-fluorouracil (5-FU) and leucovorin (Table). Response was assessed 8 weeks after completion of chemoradiation based on clinical, radiologic, and endoscopic factors. Patients with no residual mass or ulcer on clinical and endoscopic evaluation, and no persistent tumor on radiologic studies, were considered to have a cCR. Of the 361 patients treated with chemoradiation, 122 (34%) had a cCR; these patients were followed on a regular basis with physical examination, proctoscopy, biopsies of suspicious areas, and carcinoembryonic antigen (CEA) levels. Ninety-nine (27%) patients had sustained complete tumor regression for at least 1 year, and were further analyzed. Among these 99 patients, the clinical T category was unknown in 21%, was T2 in 14%, was T3 in 61%, and was T4 in 4%, while the clinical N category was unknown in 21%, N0 in 57%, and N+ in 22%. Endoluminal recurrences developed in only 5 (5%) patients, of whom 3 underwent salvage APR or low anterior resection (LAR), and 2 declined radical surgery and underwent either local excision or brachytherapy. No patient developed a pelvic regional recurrence, and 8 (8%) patients developed metastatic disease, including one with simultaneous endoluminal and distant failure. With a mean follow-up of 60 months, the 5-year disease-free survival (DFS) rate was 85%, and the 5-year OS rate was 93%. This study suggests that with careful assessment of response and close follow-up, a watch-and-wait approach could be a viable treatment option for rectal cancer patients after chemoradiation. Limitations of this study include the absence of preclinical staging in a significant proportion of patients and the inclusion of some early-stage patients. We should also emphasize that of the patients who had an initial cCR, 19% experienced recurrence within the first year and were excluded from further analysis.
A prospective cohort study in the Netherlands examined the watch-and-wait approach among clinical complete responders after chemoradiation.[19] Between 2004 and 2010, a total of 192 patients were treated with radiation therapy at a dose of 50.4 Gy, along with concurrent capecitabine. The investigators followed their standard practice and administered chemoradiation only to patients with locally advanced rectal cancer, which they defined as T4 tumor, or T3 tumor with a threatened/involved circumferential resection margin (CRM) or more than three involved nodes, or distal tumor with involved nodes. Response was assessed 6 to 8 weeks after patients were treated with chemoradiation, using magnetic resonance imaging (MRI) and endoscopy. Patients had to fulfill all of the following criteria in order to be considered to have a cCR: no residual tumor or tumor fibrosis only on MRI, no suspicious lymph nodes on MRI, no residual tumor or only small residual ulcer or scar at endoscopy, negative biopsies, and no palpable tumor on digital rectal examination (DRE). Twenty-one patients (11%) were found to have a cCR based on these criteria. Among these patients, the clinical T category was T1 in 5%, T2 in 24%, T3 in 62%, and T4 in 10%; 71% had node-positive disease. These patients were then followed under an intensive monitoring schedule, including endoscopy, MRI, DRE, and CEA levels four times during the first year and two to four times annually thereafter, as well as CT scans two times in the first year and annually thereafter. Of the 21 patients, only 1 (5%) developed an endoluminal recurrence and was treated with transanal endoscopic microsurgery. With a mean follow-up of 25 months, the 2-year DFS was 89% and the 2-year OS was 100%. In comparison, a control group of 20 patients with pCRs after chemoradiation and TME had 2-year DFS of 93% and 2-year OS of 91%. Moreover, patients treated with the watch-and-wait approach had significantly improved bowel function scores compared with the control patients, who underwent TME. This study shows that a nonoperative approach could yield excellent oncologic and functional results in carefully selected patients with cCR. However, intensive assessment is necessary to determine which patients have cCR, and only a small proportion of patients who undergo chemoradiation would qualify for the nonoperative approach. It should be noted that only patients with relatively advanced features on MRI were selected for chemoradiation in this study; the rate of cCR could potentially have been higher for patients with less advanced tumors. The mean follow-up of this study was only about 2 years, and longer follow-up will be necessary to confirm that this approach results in acceptable DFS and OS rates. Because only 21 patients were treated with the non-operative approach, caution must be exercised in generalizing the results from this study. Lastly, this study was done in a high-volume academic center, and it remains unknown whether this approach could be applied equally successfully in other settings.
An ongoing phase II multicenter trial in the United Kingdom is evaluating the watch-and-wait approach toward management of rectal cancer.[20] Patients receive radiation therapy at a minimum dose of 50.4 Gy, with concurrent capecitabine, and they undergo CT and MRI 4 weeks after chemoradiation. Those with a good partial response or a cCR are eligible for deferral of surgery, while those with biopsy-proven regrowth or progression undergo surgery. Follow-up studies include positron emission tomography (PET)-CT. A preliminary report on the results of this study described 19 patients, of whom 10 (53%) remain in complete remission, 6 have undergone salvage surgery, and 3 have declined surgery. The accrual goal of the trial is 59 patients, with the predefined target for considering this approach successful being the ability of 11 patients to avoid surgery. Completion of this trial and publication of the final results are pending.
A smaller study from the United Kingdom also evaluated the nonoperative approach for patients with rectal cancer.[21] Forty-nine patients underwent chemoradiation for rectal cancer at a single center. Similar to the Dutch study, only patients with threatened CRM or involved nodes on MRI were considered for chemoradiation. Of the 49 patients, 12 had significant regression on MRI after chemoradiation, including 6 patients who also had no evidence of residual disease on examination under anesthesia and biopsy. These 6 patients were managed conservatively without surgery. Follow-up evaluations included repeat examination under anesthesia, as well as CEA levels, PET-CT, and MRI. At a mean follow-up of 25.5 months, all 6 patients had no evidence of disease. Although this is a small study with limited follow-up, it adds to the data supporting a watch-and-wait approach for selected patients.
Investigators at the Memorial Sloan-Kettering Cancer Center conducted a retrospective study of nonoperative management of rectal cancer patients with cCR.[22] Thirty-two patients managed with this approach were identified by surgeon recall. Patients were treated with a median radiation dose of 50.4 Gy. Unlike the previous studies, the timing and methods of response assessment were not standardized, since this was a retrospective evaluation.
Patients were evaluated for response 4 to 10 weeks after chemoradiation, and response was assessed with a combination of DRE, endoscopy, and selective biopsy. All 32 patients had no palpable tumor on DRE, and no visible lesion other than a flat scar on endoscopy. Patients were followed with physical examination and sigmoidoscopy every 3 months for a year, and then every 4 to 6 months thereafter. Adjuvant chemotherapy was given to 53% of patients. Six patients developed local recurrence, including 3 who also developed metastasis.
All 6 patients with local recurrence underwent salvage surgery with either LAR or APR. There was no significant difference in the rates of metastasis, DFS, or OS in patients treated with the non-operative approach, compared with a control group of 57 patients with pCR after chemoradiation and TME. In contrast, the 2-year local recurrence rate was significantly higher in patients treated with the nonoperative approach, compared with the control group (21% vs 0%). The rate of local recurrence in this series appears much higher than the local recurrence rates reported in the studies from Brazil and the Netherlands,[17-19] and raises concern about the watch-and-wait approach. However, unlike those studies and as previously noted, the Memorial Sloan-Kettering Cancer Center study identified patients based on physician recall, which could be susceptible to biases. Moreover, because it was retrospective, there were no standardized, predefined methods for patient selection, response assessment, or follow-up.
Investigators from the Mount Vernon Hospital in the United Kingdom reported a retrospective study from a prospectively maintained database of 266 patients treated with preoperative chemoradiation for rectal cancer, between 1993 and 2005.[23] A non-operative approach was pursued in 10 patients, who had cCRs 6 to 8 weeks after chemoradiation therapy, based on complete resolution on DRE, as well as MRI findings from 1997 onwards. Surgery was not performed in these patients because of severe patient comorbidities or patient choice. Six (60%) of the 10 patients developed pelvic recurrences, and all 6 also developed simultaneous or subsequent metastatic disease. The median time to local progression was 20 months. Although most patients in this series had concurrent illnesses, 6 died from recurrent rectal cancer. While this was also a small, retrospective study with inherent limitations and a lack of standardized methods for response assessment and follow-up, it, too, raises concerns about watch-and-wait strategies in rectal cancer.
A number of other studies have also shown high rates of local recurrence with the watch-and-wait approach. A group of investigators from Brazil reported on 52 patients with mid-rectal or low rectal adenocarcinoma treated with radiation therapy, mostly at a dose of 50.4 Gy, with concurrent 5-FU and leucovorin.[24] About 3 to 4 weeks after receiving chemoradiation therapy, patients were evaluated with proctoscopy and biopsy; 10 patients (19%) were found to have cCR, defined as complete regression on proctoscopy and negative biopsy. These 10 patients with cCR were followed without surgery, but 8 (80%) subsequently developed local recurrence, at a mean time of 6 months post treatment. A prior prospective trial from this group in Brazil also showed an unacceptably high rate of local recurrence in patients with cCR after chemoradiation.[25] Five of six patients (83%) with cCR developed local recurrence, with the cCR lasting a mean of 11 months. In a study from a tertiary care center in India, 23 patients with a cCR after chemoradiation were followed without surgery.[26] With a median follow-up of 6 years, 7 patients (30%) developed isolated local recurrence.
In summary, the best results with the watch-and-wait approach appear to be from studies that have used clearly defined and standardized methods for patient selection, response assessment, and follow-up, such as the Brazilian (Habr-Gama) series and the Dutch prospective trial.[17-19] Preliminary results from the UK phase II trial and another smaller UK study also support this approach.[20,21] In contrast, in multiple other clinical studies, patients treated with the nonoperative approach appear to have had unacceptably high rates of local failure.[22-26]
Assessment of Response
The success of the watch-and-wait strategy is contingent upon accurate assessment of a given patient’s response to chemoradiation. Our current methods of assessing tumor response have limited accuracy, and the presence of a cCR does not necessarily imply that a pCR has occurred. In a retrospective study of 488 patients from Memorial Sloan-Kettering Cancer Center, 19% had a cCR to preoperative chemoradiation, based on DRE and proctoscopy.[27] However, only 25% of patients with cCRs had pCRs, while the majority had persistent tumor foci on pathologic examination of the surgical specimen. Moreover, patients with complete response at the primary tumor site may sometimes have persistent nodal involvement. In the Memorial Sloan-Kettering Cancer Center study, for example, 15% of clinical complete responders with pathologic T0 disease had pathologic node-positive disease.[27] Similarly, in a study from The University of Texas MD Anderson Cancer Center, 9% of patients with pathologic T0 disease after chemoradiation had pathologic nodal involvement.[28]
Advances in imaging techniques could augment the accuracy of response assessment. MRI is now widely used for initial staging of rectal cancer, and it could potentially also help in evaluating response to treatment. Some of the more recent studies discussed above have already used MRI for the evaluation of tumor response.[19-21,23] Investigators from the Netherlands have identified four distinct MRI patterns associated with persistent complete response: normalized rectal wall, full-thickness fibrosis, minimal fibrosis, and specular fibrosis.[29] Interestingly, in their study only 26% of patients with cCR had a normalized rectal wall, while the majority had a pattern of fibrosis. Investigators from the United Kingdom have also reported MRI parameters associated with post-treatment T stage and tumor regression grade.[30] A study from Brazil evaluated PET-CT after chemoradiation in 99 patients, and found that PET-CT had 85% accuracy and 93% sensitivity in detecting residual cancer after chemoradiation.[31] The use of these imaging studies needs to be validated in additional prospective trials.
A structured, predefined method of response assessment and follow-up will be critical for a watch-and-wait strategy. Ideally, response assessment should include at least DRE, endoscopy with biopsies when appropriate, and MRI. The role of PET-CT in evaluating response needs to be evaluated further. There should also be a structured, predefined method for the “watch” part of watch-and-wait, with regular follow-up assessments. Many patients with an initial cCR have early local recurrences, and it is imperative that these recurrences be detected in time for salvage surgery to remain an option. Follow-up assessments should include at least DRE, endoscopy with biopsies when appropriate, CEA level, and MRI. The optimal timing and frequency of follow-up assessments need to be further investigated.
Enhancement of Response
The rate of cCR is relatively low after radiation therapy with concurrent 5-FU or capecitabine, which limits the number of rectal cancer patients eligible for a watch-and-wait approach. For example, only 27% of patients receiving chemoradiation had sustained complete response in the Habr-Gama series, and only 11% of patients had a cCR after chemoradiation in the Dutch trial.[17,19] Multiple clinical trials have evaluated the addition of chemotherapeutic and biologic agents to standard fluoropyrimidine-based chemoradiation, in an effort to improve the response rate. The addition of oxaliplatin to chemoradiation did not increase pathologic response rates in three randomized trials.[12,32,33] In phase II trials, while the addition of bevacizumab to chemoradiation resulted in pathologic response rates of 16% to 32%, it also increased surgical complications.[34-39] The addition of cetuximab to chemoradiation appeared to lower response rates rather than increasing them.[40-43] Hence, radiation therapy concurrent with 5-FU or capecitabine remains the standard for chemoradiation. It is hoped that future trials with newer targeted agents will lead to increased response rates, thereby expanding the scope of the watch-and-wait approach. In addition, radiotherapy dose escalation with techniques such as intensity-modulated radiation therapy (IMRT) could potentially increase tumor response, although the role of radiation dose escalation will also need to be studied in prospective trials.[44]
Patient selection based on biologic and genomic factors may help to identify patients who are most likely to respond to chemoradiation. For example, increased expression of thymidylate synthase and apoptosis protease-activating factor 1, and decreased expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR), have been associated with increased response to chemoradiation.[45-49] Furthermore, a systems biology–based multigene expression model of radiosensitivity has been developed and validated in a cohort of rectal cancer patients.[50,51] These innovative approaches may help clinicians to select the patients who are most appropriately managed with the watch-and-wait approach.
Conclusions
A number of recent prospective and retrospective studies have evaluated the watch-and-wait approach for rectal cancer patients who have a cCR after chemoradiation. The watch-and-wait strategy could potentially reduce treatment-related toxicity and improve functional outcomes in selected rectal cancer patients. Since cCR does not necessarily imply that the patient has had a pCR, caution must be exercised in pursuing nonoperative approaches. Some studies have shown excellent oncologic outcomes with the watch-and-wait strategy, while others have reported high rates of local recurrence. Careful patient selection, systematic methods of response assessment, and close follow-up will be critical to the success of the nonoperative approach. Ideally, response assessment and follow-up should include DRE, MRI, and endoscopy, with biopsies performed as appropriate. Currently only a small proportion of patients undergoing chemoradiation show cCR. More effective chemoradiation regimens, perhaps with molecular and targeted agents, will be needed in order to apply the watch-and-wait strategy to a broader patient population. Advances in imaging technology and in our knowledge of biologic and genomic factors associated with tumor response may facilitate patient selection for non-operative approaches. While the watch-and-wait approach appears intriguing, additional clinical studies are warranted to establish its safety and efficacy. Ideally, watch-and-wait strategies should be pursued within the context of prospective clinical trials.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Heald RJ. Total mesorectal excision is optimal surgery for rectal cancer: a Scandinavian consensus. Br J Surg. 1995;82:1297-9.
2. Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926-33.
3. van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-82.
4. Khrizman P, Niland JC, ter Veer A, et al. Postoperative adjuvant chemotherapy use in patients with stage II/III rectal cancer treated with neoadjuvant therapy: a National Comprehensive Cancer Network analysis. J Clin Oncol. 2013;31:30-8.
5. Callender GG, Das P, Rodriguez-Bigas MA, et al. Local excision after preoperative chemoradiation results in an equivalent outcome to total mesorectal excision in selected patients with T3 rectal cancer. Ann Surg Oncol. 2010;17:441-7.
6. Garcia-Aguilar J, Shi Q, Thomas CR, Jr, et al. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol. 2012;19:384-91.
7. Simunovic M, Jacob S, Coates AJ, et al. Outcomes following a limited approach to radiotherapy in rectal cancer. Br J Surg. 2011;98:1483-8.
8. Taylor FG, Quirke P, Heald RJ, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711-9.
9. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-40.
10. Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-23.
11. Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620-5.
12. Roh M, Yothers G, O’ Connell M, et al. The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R-04. J Clin Oncol. 2011;29(suppl):abstr 3503.
13. Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124-30.
14. Wang Y, Cummings B, Catton P, et al. Primary radical external beam radiotherapy of rectal adenocarcinoma: long term outcome of 271 patients. Radiother Oncol. 2005;77:126-32.
15. Gerard JP, Chapet O, Ramaioli A, Romestaing P. Long-term control of T2-T3 rectal adenocarcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys. 2002;54:142-9.
16. Lim L, Chao M, Shapiro J, et al. Long-term outcomes of patients with localized rectal cancer treated with chemoradiation or radiotherapy alone because of medical inoperability or patient refusal. Dis Colon Rectum. 2007;50:2032-9.
17. Habr-Gama A, Perez RO, Proscurshim I, et al. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. J Gastrointest Surg. 2006;10:1319-28.
18. Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711-7.
19. Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633-40.
20. Yu S, Brown G, Heald R, et al. Deferral of rectal surgery following a continued response to preoperative chemoradiotherapy (watch and wait) study: a phase II multicenter study in the United Kingdom. J Clin Oncol. 2011;29(suppl 4):abstr 489.
21. Dalton RS, Velineni R, Osborne ME, et al. A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis. 2012;14:567-71.
22. Smith JD, Ruby JA, Goodman KA, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256:965-72.
23. Hughes R, Harrison M, Glynne-Jones R. Could a wait and see policy be justified in T3/4 rectal cancers after chemo-radiotherapy? Acta Oncol. 2010;49:378-81.
24. Nakagawa WT, Rossi BM, de O Ferreira F, et al. Chemoradiation instead of surgery to treat mid and low rectal tumors: is it safe? Ann Surg Oncol. 2002;9:568-73.
25. Rossi BM, Nakagawa WT, Novaes PE, et al. Radiation and chemotherapy instead of surgery for low infiltrative rectal adenocarcinoma: a prospective trial. Ann Surg Oncol. 1998;5:113-8.
26. Seshadri RA, Kondaveeti SS, Jayanand SB, et al. Complete clinical response to neoadjuvant chemoradiation in rectal cancers: can surgery be avoided? Hepatogastroenterology. 2013 May 1. [Epub ahead of print]
27. Hiotis SP, Weber SM, Cohen AM, et al. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg. 2002;194:131-5.
28. Park IJ, You YN, Skibber JM, et al. Comparative analysis of lymph node metastases in patients with ypT0-2 rectal cancers after neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2013;56:135-41.
29. Lambregts DM, Maas M, Bakers FC, et al. Long-term follow-up features on rectal MRI during a wait-and-see approach after a clinical complete response in patients with rectal cancer treated with chemoradiotherapy. Dis Colon Rectum. 2011;54:1521-8.
30. Patel UB, Brown G, Rutten H, et al. Comparison of magnetic resonance imaging and histopathological response to chemoradiotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2012;19:2842-52.
31. Perez RO, Habr-Gama A, Gama-Rodrigues J, et al. Accuracy of positron emission tomography/computed tomography and clinical assessment in the detection of complete rectal tumor regression after neoadjuvant chemoradiation: long-term results of a prospective trial (National Clinical Trial 00254683). Cancer. 2012;118:3501-11.
32. Gerard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638-44.
33. Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773-80.
34. Landry JC, Feng Y, Cohen SJ, et al. Phase 2 study of preoperative radiation with concurrent capecitabine, oxaliplatin, and bevacizumab followed by surgery and postoperative 5-fluorouracil, leucovorin, oxaliplatin (FOLFOX), and bevacizumab in patients with locally advanced rectal cancer: ECOG 3204. Cancer. 2013;119:1521-7.
35. Spigel DR, Bendell JC, McCleod M, et al. Phase II study of bevacizumab and chemoradiation in the preoperative or adjuvant treatment of patients with stage II/III rectal cancer. Clin Colorectal Cancer. 2012;11:45-52.
36. Kennecke H, Berry S, Wong R, et al. Pre-operative bevacizumab, capecitabine, oxaliplatin and radiation among patients with locally advanced or low rectal cancer: a phase II trial. Eur J Cancer. 2012;48:37-45.
37. Dipetrillo T, Pricolo V, Lagares-Garcia J, et al. Neoadjuvant bevacizumab, oxaliplatin, 5-fluorouracil, and radiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82:124-9.
38. Crane CH, Eng C, Feig BW, et al. Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2010;76:824-30.
39. Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009;27:3020-6.
40. Machiels JP, Sempoux C, Scalliet P, et al. Phase I/II study of preoperative cetuximab, capecitabine, and external beam radiotherapy in patients with rectal cancer. Ann Oncol. 2007;18:738-44.
41. Rodel C, Arnold D, Hipp M, et al. Phase I-II trial of cetuximab, capecitabine, oxaliplatin, and radiotherapy as preoperative treatment in rectal cancer. Int J Radiat Oncol Biol Phys. 2008;70:1081-6.
42. Bengala C, Bettelli S, Bertolini F, et al. Epidermal growth factor receptor gene copy number, K-ras mutation and pathological response to preoperative cetuximab, 5-FU and radiation therapy in locally advanced rectal cancer. Ann Oncol. 2009;20:469-74.
43. Weiss C, Arnold D, Dellas K, et al. Preoperative radiotherapy of advanced rectal cancer with capecitabine and oxaliplatin with or without cetuximab: a pooled analysis of three prospective phase I-II trials. Int J Radiat Oncol Biol Phys. 2010;78:472-8.
44. Appelt AL, Ploen J, Vogelius IR, et al. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:74-80.
45. Kikuchi M, Mikami T, Sato T, et al. High Ki67, Bax, and thymidylate synthase expression well correlates with response to chemoradiation therapy in locally advanced rectal cancers: proposal of a logistic model for prediction. Br J Cancer. 2009;101:116-23.
46. Lamas MJ, Duran G, Gomez A, et al. X-ray cross-complementing group 1 and thymidylate synthase polymorphisms might predict response to chemoradiotherapy in rectal cancer patients. Int J Radiat Oncol Biol Phys. 2012;82:138-44.
47. Negri FV, Campanini N, Camisa R, et al. Biological predictive factors in rectal cancer treated with preoperative radiotherapy or radiochemotherapy. Br J Cancer. 2008;98:143-7.
48. Edden Y, Wexner SD, Berho M. The use of molecular markers as a method to predict the response to neoadjuvant therapy for advanced stage rectal adenocarcinoma. Colorectal Dis. 2012;14:555-61.
49. Toiyama Y, Inoue Y, Saigusa S, et al. Gene expression profiles of epidermal growth factor receptor, vascular endothelial growth factor and hypoxia-inducible factor-1 with special reference to local responsiveness to neoadjuvant chemoradiotherapy and disease recurrence after rectal cancer surgery. Clin Oncol (R Coll Radiol). 2010;22:272-80.
50. Eschrich SA, Pramana J, Zhang H, et al. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys. 2009;75:489-96.
51. Eschrich S, Zhang H, Zhao H, et al. Systems biology modeling of the radiation sensitivity network: a biomarker discovery platform. Int J Radiat Oncol Biol Phys. 2009;75:497-505.