Which Larynx-Preservation Strategy Would Be Best for a Patient With Resectable, Locally Advanced, Hypopharynx Squamous Cell Carcinoma?
A 57-year-old man with a heavy smoking and alcohol consumption history, but no comorbidities, presented with pharyngeal pain to his primary care physician’s office. He received empirical treatment with oral antibiotics and experienced partial improvement. A couple of months later, he developed a left cervical mass with progressive growth. A head and neck CT scan revealed a hypopharyngeal tumor. Results of a tumor biopsy indicated an ulcerated, moderately differentiated squamous cell carcinoma (SCC) with lymphovascular invasion. The patient was sent to our institution for treatment.
Sobrevilla-Moreno Staff Physician; Medical Oncologist; Department of Medical Oncology, Head & Neck Cancer; Instituto Nacional de Cancerologia.

Altamirano-García staff physician Radiation Oncologist; Department of Radiation Oncology, Head & Neck Cancer; Instituto Nacional de Cancerología, Mexico City, México.

Granados-Garcia Head of the Head & Neck Surgical Department; Surgical Oncologist; Department of Surgical Oncology, Head & Neck Cancer; Instituto Nacional de Cancerologia.

Garcilazo-Reyes Medical Oncology Fellow; Department of Medical Oncology; Instituto Nacional de Cancerologia.

Key Points
• LP strategies should be considered only in patients with resectable stage III/IV larynx and hypopharynx SCC, ideally those who have a functional larynx.
• The choice of treatment must be discussed by a multidisciplinary team, and the patient must have an active role in making the decision.
• CRT and IC-RT are both approved larynx-preservation approaches.
• IC-RT has demonstrated high rates of LP without decreasing DFS or OS, compared with surgery.
• CRT has demonstrated better LP rates, as well as local control and LRC, compared with IC-RT, and it should be considered a standard of care.
The Case
A 57-year-old man with a heavy smoking and alcohol consumption history, but no comorbidities, presented with pharyngeal pain to his primary care physician’s office. He received empirical treatment with oral antibiotics and experienced partial improvement. A couple of months later, he developed a left cervical mass with progressive growth. A head and neck CT scan revealed a hypopharyngeal tumor. Results of a tumor biopsy indicated an ulcerated, moderately differentiated squamous cell carcinoma (SCC) with lymphovascular invasion. The patient was sent to our institution for treatment.
At the first visit, the patient complained of pharyngeal pain, hoarseness, mild pain of the bilateral cervical matted lymph nodes, mild dysphonia, and no swallowing issues. He did not mention dysphagia or weight loss, and he had quit smoking 2 months before evaluation. In the physical examination, he was found to have COG 1, with bilateral cervical matted lymph nodes; the largest one, measuring 7 cm x 6 cm, was located on the right side of the neck.
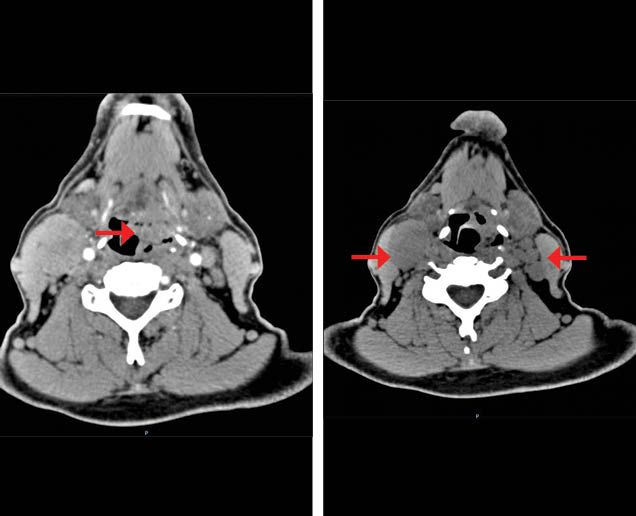
The nasofibrolaryngoscopy described a tumor at the hypopharynx that invaded the epiglottis and the posterior pharyngeal wall, with fixation of the right hemilarynx. An endoscopy ruled out extension to the esophagus. The CT scan described the presence of a tumor at the hypopharyngeal level with transglottic extension. It also revealed bilateral cervical matted lymph nodes (Figure 1a). Chest CT scan ruled out distant metastases; the complete blood count and blood chemistry were normal. The patient was given a diagnosis of a stage IVB (T3N3M0) hypopharynx SCC.
Figure 1A. Basal contrast enhanced CT scan shows a tumor with heterogeneous enhancement located in the pharyngomucosal space (hypopharynx) with lost interface with the lateral portion of the glottis (left). Right cervical matted nodes in level IIA and multiple left cervical nodes (right).
The case was discussed in a multidisciplinary board. Considering the characteristics of the patient and the disease, both a total laryngectomy with neck dissection and a larynx preservation (LP) strategy were proposed. The options were discussed with the patient, who refused to have the surgical treatment.
Question:
In this patient, which LP treatment option(s) could be considered?
A. Definitive chemoradiotherapy (CRT).
B. Definitive bioradiotherapy (BRT) with cetuximab.
C. Induction chemotherapy (IC) with cetuximab followed by CRT.
D. IC and then, according to response, consider CRT vs surgery.
Answer:
A and D are both suitable options.
Discussion
Head and neck squamous cell carcinoma (HNSCC) represented 705,781 of new cancer cases and caused 358,144 deaths worldwide in 2018.1 HNSCC varies in incidence according to anatomical site.1 Stage distribution also varies among anatomical sites2,3 and by geographical location, with some countries describing an incidence of locally advanced (LA) disease in up to 70% of their HNSCC patients.4 Depending on the site of the primary tumor, recurrence rates in LA disease are between 25% and 50%.5
Hypopharynx SCC is a rare disease that accounts for 3% to 5% of all HNSCC. Consequently, treatment decisions are frequently based on data from other sites (ie, larynx). Most of these patients will receive a diagnosis of LA disease and have a poor prognosis.6
Patients with SCC of the larynx and/or hypopharynx can be treated with combinations of chemotherapy and radiotherapy (RT) instead of surgery, in cases when the tumor is resectable but resection would involve losing a crucial organ or function. The definition of LP has changed over time. The first trials evaluating this procedure focused on preserving “the larynx in place.” With time, function preservation was also considered, including the avoidance of such procedures as long-term tracheotomy or feeding-tube placement.7
Chemoradiotherapy for Resectable LA SCC of Larynx and Hypopharynx
Definitive chemoradiotherapy (CRT) is considered a standard treatment for LA HNSCC. In the Chemotherapy in Head and Neck Cancer meta-analysis, concurrent treatment with RT and chemotherapy was shown to be the most effective treatment option, compared with induction chemotherapy (IC)-RT or RT alone, with a decrease in the risk of death of 19% and an absolute benefit in overall survival (OS) of 6.5% at 5 years,8 which persisted when analyzed by tumor site.9
In terms of LP, the RTOG 91-11 trial randomized patients with stage III and IV (low-volume T4 tumors) SCC of the larynx to receive IC-RT, CRT, or RT alone. Only patients with a complete response (CR) or partial response (PR) after 3 cycles of IC were treated with RT. The control arm was the IC-RT arm.10 After a 10-year follow-up, there was no difference between the IC-RT and CRT arms in terms of laryngectomy-free survival (HR, 1.05; 95% CI, 0.83-1.34; P = .68). The rates of laryngeal preservation, local control (LC), and locoregional control (LRC) were superior for the CRT arm compared with the IC-RT arm, and they were not different when comparing the IC-RT and the RT-alone arms. There were no differences in distant disease control, disease-free survival (DFS), or OS between IC-RT and CRT, although a tendency for better survival in the IC-RT arm compared with the CRT arm was observed (HR, 1.25; 95% CI, 0.98-1.61; P = .08). In terms of impaired speech and swallowing dysfunction, rates remained similar between the groups.11
To our knowledge, the only randomized phase 3 study that has compared CRT with IC in SCC of the hypopharynx was conducted by Prades et al12; it compared CRT with cisplatin to IC with cisplatin plus 5-fluorouracil (PF) followed by conventional RT in patients with stage T3 disease with a fixed hemilarynx. The rates of LP at 2 years were 67.5% for IC and 92.0% for CRT (P = .016). There was no difference between the arms in terms of DFS or OS.
It must be taken into account that associated toxicity is always greater when chemotherapy is given concurrently with RT than when RT alone is given. Nevertheless, with modern radiotherapy techniques, acute G4 mucositis occurs in less than 5% of the patients; chronic G3 toxicity in the esophagus and mucosa, and xerostomia, each occur in about 4%.13
A caveat of these studies is that, until the 2000s, they all utilized outdated RT techniques, with heterogeneous fractionation. Modern RT techniques have demonstrated an impact on LC rates in treating nasopharynx and oropharynx SCC,13,14 as well as an impact on tolerance; furthermore, reduced long-term complications associated with modern RT techniques could be associated with decreased rates of nononcological deaths, which were seen in patients treated with older RT techinques.15 The use of modern techniques could lead to better survival with CRT than with IC-RT, but no prospective data are yet available.
Induction Chemotherapy for Resectable LA HNSCC of the Larynx and Hypopharynx
Clinical guidelines recommend surgery or CRT (for LP purposes) as treatment options for resectable disease; nevertheless, most of the guidelines do not consider IC as a standard treatment.16-18
Several studies have explored the use of IC for LP purposes, all evaluating response after a certain number of IC cycles. A PR, defined as reduction of the primary tumor by at least 50%, was considered as a cutpoint to decide whether a patient could receive RT or should be treated with surgery.
The first randomized trial that evaluated IC for LP was the Department of Veterans Affairs Laryngeal Cancer study.19 Patients with resectable stage III/IV SCC of the larynx were randomized to total laryngectomy or to PF IC; after 2 cycles of IC, patients with a PR were treated with 1 more cycle of IC followed by RT. Of the patients in the PF IC arm, 85% achieved a PR and were treated with RT. The larynx was preserved in 64% of patients treated with IC. Stage IV disease and T4 were both associated with greater odds of a total laryngectomy after IC-RT. There was no difference between the arms in terms of OS or DFS. Patterns of recurrence differed between the groups: Patients receiving IC-RT had significantly more local recurrences than those in the surgery arm (12% vs 2%; P = .001) but fewer distant recurrences (17% vs 12%, respectively; P = .001).
The European Organisation for Research and Treatment of Cancer conducted a similar trial in patients with hypopharyngeal SCC. This trial randomized patients with T1-T4, N0-N2b, and operable N3 disease to receive IC with PF followed by RT or surgery. Response rates were evaluated after each cycle of IC. Only patients who achieved a CR after 2 or 3 cycles of IC were treated with RT; this condition was met by 61% of patients and they received RT.20 In a 10-year follow-up, there were no differences in first progression, locoregional failures, or distant metastases between the RT and surgery arms. The 5- and 10-year disease-specific survival rates with preserved larynx for the IC arm were 40.5% and 26.97%, respectively. Noninferiority of OS (2.1 years for surgery vs 3.7 years for IC; HR, 0.88; P = .002) was achieved.21
The addition of a taxane to the IC regimen (TPF) was analyzed in the GORTEC 2000-01 trial. Patients with resectable LA larynx and hypopharynx patients with stage III or IV disease were randomly assigned to receive TPF or PF, followed by RT in those patients with a CR or a PR and recovery of normal cordal mobility. Rates of PR and CR were higher in the TPF arm. Subsequently, 76.4% and 61.2% of patients in the TPF and PF arms, respectively, received RT. The 3-year LP, the primary end point, was 70.3% for the TPF arm and 57.5% for the PF regimen (P = .03) and was preserved long-term.22 The 10-year larynx dysfunction–free survival rates (death, local relapse, total or partial laryngectomy, tracheotomy at 2 years or later, or feeding tube at 2 years or later) were 63.7% (95% CI, 0.52-0.74) and 37.2% (95% CI, 0.24-0.52; P = .001), in the TPF and PF arms, respectively. No differences in OS, DFS, or LRC rates were detected between the arms. These results were confirmed with a meta-analysis, which described a survival benefit for the TPF regimen over PF.23
With these data, we conclude that IC-RT can be an option as a LP strategy for resectable LA HNSCC in the larynx and/or hypopharynx, increasing the rate of LP and function, without negatively affecting OS, DFS or LRC. Achieving a PR with IC seems to be a predictor of response to RT. The regimen of choice for the IC-RT strategy is TPF.
Nevertheless, several questions remain: Is IC-RT better than or equal to CRT alone? Which is the best strategy for patients with a good response after IC: RT alone or CRT? If so, what is the best drug to combine with RT in CRT: cisplatin, carboplatin, or cetuximab?
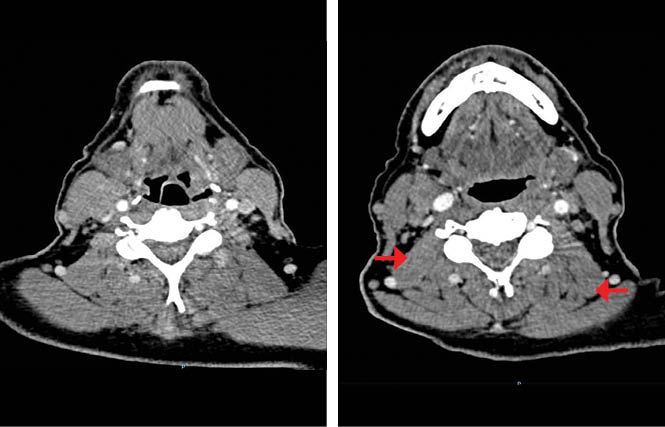
Our patient began induction chemotherapy based on the TPF regimen. He received 2 cycles and experienced grade 2 toxicities that did not require treatment delays. A subsequent CT scan indicated decreases of the primary tumor and the cervical matted nodes, equivalent to a PR with a greater than 50% reduction in tumor size (Figure 1b).
Figure 1B. Post induction chemotherapy (IC) contrast enhanced CT scan shows a partial response of the tumor located in the hypopharynx (left). Decrease in size of the right matted lymph nodes and the left lymph nodes (right).
The patient was reevaluated in the tumor board, and it was decided to continue treatment with CRT as definitive therapy. The patient was treated with conventional fractionation, sequential,intensity modulated RT, 70 Gy (46 Gy/23 fractions + 24 Gy/12 fractions) for 7 weeks. Concurrent chemotherapy consisted of 3 cycles of high-dose cisplatin (100 mg/m2) every 3 weeks. The patient developed G2 dysphonia, dysgeusia, xerostomia, nausea, and radioepithelitis, as well as grade 1 mucositis and dysphagia.
IC Followed by CRT in Resectable HNSCC of the Larynx and Hypopharynx
Little prospective evidence exists for this treatment approach. In fact, to our knowledge, no prospective randomized phase 3 trials directly compare IC-CRT with CRT for LP.
The TREMPLIN trial is a phase 2 randomized trial that compared CRT vs bioradiotherapy with cetuximab, after 3 cycles of IC with TPF, in patients with resectable LA SCC of the larynx. A total of 76% of patients completed 3 cycles of TPF; 24% dropped out after IC, mostly due to toxicity. Of the patients randomized to the CRT arm, 84% received at least 2 cycles of cisplatin, and 71% of those in the cetuximab arm completed 7 doses. There was no difference in G3/4 toxicity between cisplatin and cetuximab in respect to mucositis; skin reactions were more frequent with cetuximab. Patients in the cisplatin arm required more dose modifications due to acute toxicities. Local failures were less frequent with cisplatin (8.3% vs 14.3%). Because of the number of dropouts, the primary end point of LP at 3 months was not evaluable. The analysis did not show a difference between the 2 arms, and the LP rate at 3 months was very similar to that of patients treated with IC-RT alone in the GORTEC 2000-01 trial (LP for CRT, 95%; 95% CI, 86%-98%; LP for RT-cetuximab, 93%; 95% CI, 83%-97%).24
Other studies have focused on using 1 cycle of IC to identify patients who would be good candidates for CRT. A prospective 1-arm trial evaluated this strategy in patients with resectable SCC of the larynx. After 1 cycle of PF, 75% of patients achieved greater than 50% response, and 70% achieved LP after cisplatin-based CRT.25 In a cohort at the University of Michigan, patients who were treated with IC for 1 cycle followed by CRT had better disease-specific survival compared with those treated with CRT alone (HR, 0.48; 95% CI, 0.29-0.80).26 These studies suggest that just 1 cycle of IC may be enough to select patients for CRT, compared with 3 cycles, and the strategy may improve tolerance and adherence to treatment.
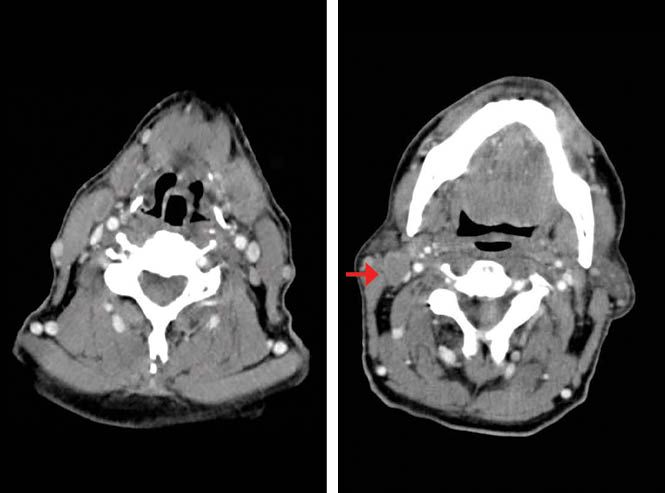
Ten weeks after the patient finished CRT, a CT scan reported persistence of cervical adenopathies and CR of the primary tumor (Figure 1c). A laryngoscopy described edema of the lateral walls of the hypopharynx, base of the tongue, and uvula, without evidence of tumor. The pathological findings revealed unspecific chronic inflammatory changes.
Figure 1C. Post chemoradiotherapy (CRT) contrast enhanced CT scan shows postradiotherapy changes in the hypopharynx, no evidence of the primary tumor (left). Right cervical matted nodes (right).
Due to the persistence of lymph node disease with a complete primary response and without evidence of metastatic disease, a bilateral selective neck dissection, levels II to IV, was performed. The pathology findings were metastatic SCC in 3 of 12 dissected right lymph nodes and in 4 of 13 left lymph nodes.
Planned or Complementary Surgery on the Neck
When a PR, versus a CR, is reached after a nonsurgical CRT approach in LA HNSCC, surgery undoubtedly improves locoregional control; in fact, most patients in studies of IC-RT who had a PR experienced a resection of persistent disease. But even with a CR, which includes negative imaging studies, planned neck dissections have been used to optimize control. In both circumstances, planned or complementary surgery improves locoregional control, prolongs OS, and possibly cures the patient.27 The specific evidence for hypopharynx SCC is limited.
N2 and N3 disease are now often treated with initial chemotherapy and RT, but planned or complementary neck dissection is used when the original lymphadenopathy was greater than 3 cm, and the primary lymph node disease has been controlled.28 Lending support to this strategy is the fact that patients with N2/N3 involvement at diagnosis who reach a CR after a CRT approach show residual tumor in up to 22% of surgical specimens.29
Two large retrospective trials evaluated the benefit of a modified radical node dissection in documented CR patients treated with chemotherapy and RT. Compared with observation, this procedure has demonstrated a benefit in LC, LRC, metastasis-free survival, and OS for N2/N3 patients.30,31
The introduction of 18-FDG-PET-CT has changed the method of evaluating CR in patients with LA HNSCC who have been treated with CRT. Compared with CT scan or MRI, PET scan has been found to be superior for neck evaluation in patients with HNSCC; it has a negative predictive value (NPV) of 97.7% at 5 years.32,33 In patients who have oropharynx SCC positive for human papillomavirus (HPV) with bulky lymphadenopathy and are undergoing CRT, observation is appropriate if CR is confirmed with a posttreatment evaluation of the neck with ultrasonography and PET scan because this combination brings the highest NPV.34,35 Nevertheless, the surveillance for patients evaluated with this technique may not be cost-effective36 and is recommended only in HPV-positive oropharynx SCC.
After surgery, the patient presented with neck stiffness, which remitted with rehabilitation exercises. He continued with surveillance visits. He continued to experience long-term grade 1 xerostomia and also developed hypothyroidism, for which he received hormonal substitution. Importantly, no swallowing or voice alterations persisted, and to date, after 5 years of follow-up, he has no clinical or radiological signs of tumor activity.
The most important steps in choosing an LP strategy involve being as sure as possible that the treatment is right for the particular patient. The decision should be weighed by a multidisciplinary team, and numerous factors must be taken into account. First, the characteristics of the tumor should be evaluated. Patients with stage III/IV resectable disease and, ideally, with a functional larynx, should be considered. Physicians must also consider the patient’s baseline characteristics. The TALK score (which considers tumor size, performance status, alcohol intake status, and baseline blood albumin) can be a good tool for discriminating whether a patient might or might not tolerate CRT.37 Another very important factor is the patient’s preference: All therapeutic options should be discussed with the patient before treatment, and they must have an active role in the decision.
In terms of the type of LP treatment to be offered, CRT is considered the current standard of care because it has shown better LP, LC, and LRC compared with IC-RT. While no difference in OS has been shown in any individual clinical trial, a large meta-analysis has indicated that CRT has an advantage in OS over IC-RT in patients with LA HNSCC. There is no standard drug to combine with RT in CRT; in our center, high-dose cisplatin is preferred.
The main advantage of IC-RT is that it can help distinguish patients who will benefit from RT from those who will need surgery. It also avoids the added toxicity generated by CRT. Response to treatment must be evaluated early, preferably after 2 cycles of IC, to define the subsequent treatment. TPF regimen is preferred over PF.
In our patient, an approach incorporating IC followed by CRT was chosen. IC followed by CRT seems to increase toxicity, and it has been difficult to prove its advantage in terms of LP, LRC, DFS, or OS. Although our patient has experienced excellent long-term control of disease, this strategy should be used only in the setting of a clinical trial. Since the publication of the results of the TREMPLIN trial, our standard of care for LP has become CRT with high-dose cisplatin.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References
1. Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 68, 394–424 (2018).
2. Laryngeal Cancer — Cancer Stat Facts. Available at: https://seer.cancer.gov/statfacts/html/laryn.html. (Accessed: 28th April 2020)
3. Oral Cavity and Pharynx Cancer — Cancer Stat Facts. Available at: https://seer.cancer.gov/statfacts/html/oralcav.html. (Accessed: 28th April 2020)
4. D’Souza, G. et al. Effect of HPV on head and neck cancer patient survival, by region and tumor site: A comparison of 1362 cases across three continents. Oral Oncol. 62, 20–27 (2016).
5. Ho, A. S. et al. Decision making in the management of recurrent head and neck cancer. Head and Neck 36, 144–151 (2014).
6. Eisele, D. W. et al. CLINICAL REVIEW CURRENT TRENDS IN INITIAL MANAGEMENT OF HYPOPHARYNGEAL CANCER: THE DECLINING USE OF OPEN SURGERY. 34, 270–281 (2010).
7. Argiris, A. & Lefebvre, J. L. Laryngeal preservation strategies in locally advanced laryngeal and hypopharyngeal cancers. Front. Oncol. 9, 1–7 (2019).
8. Pignon, J. P., Maître, A. le, Maillard, E. & Bourhis, J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 92, 4–14 (2009).
9. Blanchard, P. et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): A comprehensive analysis by tumour site. Radiother. Oncol. 100, 33–40 (2011).
10. Forastiere, A. A. et al. Concurrent Chemotherapy and Radiotherapy for Organ Preservation in Advanced Laryngeal Cancer. n engl j med 22, (2003).
11. Forastiere, A. A. et al.Long-term results of RTOG 91-11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J. Clin. Oncol. 31, 845–852 (2013).
12. Prades, J. M. et al. Randomized phase III trial comparing induction chemotherapy followed by radiotherapy to concomitant chemoradiotherapy for laryngeal preservation in T3M0 pyriform sinus carcinoma. Acta Otolaryngol. 130, 150–155 (2010).
13. Lee, N. et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: Radiation therapy oncology group phase II trial 0225. J. Clin. Oncol. 27, 3684–3690 (2009).
14. Eisbruch, A. et al. Multi-Institutional Trial of Accelerated Hypofractionated Intensity-Modulated Radiation Therapy for Early-Stage Oropharyngeal Cancer (RTOG 00-22). Int. J. Radiat. Oncol. Biol. Phys. 76, 1333–1338 (2010).
15. Petkar, I., Bhide, S., Newbold, K., Harrington, K. & Nutting, C. Dysphagia-optimised Intensity-modulated Radiotherapy Techniques in Pharyngeal Cancers: Is Anyone Going to Swallow it? Clin. Oncol. 29, e110–e118 (2017).
16. NCCN. Clinical Practice Guides in Oncology. Head and neck cancer, version 3.2019. Available at: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. (Accessed: 28th April 2020)
17. Grégoire, V., Lefebvre, J. L., Licitra, L. & Felip, E. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 21, 184–186 (2010).
18. Forastiere, A. A. et al. Use of larynx-preservation strategies in the treatment of laryngeal cancer: American society of clinical oncology clinical practice guideline update. Journal of Clinical Oncology 36, 1143–1169 (2018).
19. Wolf, G. T. et al. Induction Chemotherapy plus Radiation Compared with Surgery plus Radiation in Patients with Advanced Laryngeal Cancer. N. Engl. J. Med. 324, 1685–1690 (1991).
20. Lefebvre, J. L. et al. Larynx preservation in pyriform sinus cancer: Preliminary results of a European organization for research and treatment of cancer phase III trial. J. Natl. Cancer Inst. 88, 890–899 (1996).
21. Lefebvre, J.-L. et al. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann. Oncol. 23, 2708–2714 (2012).
22. Janoray, G. et al. Long-term results of a multicenter randomized phase III trial of induction chemotherapy with cisplatin, 5-fluorouracil, ± docetaxel for larynx preservation. J. Natl. Cancer Inst. 108, 1–7 (2016).
23. Blanchard, P. et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: An individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J. Clin. Oncol. 31, 2854–2860 (2013).
24. Lefebvre, J. L. et al. Induction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: The TREMPLIN randomized phase II study. J. Clin. Oncol. 31, 853–859 (2013).
25. Urba, S. et al. Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: A new treatment paradigm. J. Clin. Oncol. 24, 593–598 (2006).
26. Wolf, G. T. et al. Survival rates using individualized bioselection treatment methods in patients with advanced laryngeal cancer. JAMA Otolaryngol. - Head Neck Surg. 143, 355–366 (2017).
27. Patil, V. M. et al. Neoadjuvant chemotherapy followed by surgery in very locally advanced technically unresectable oral cavity cancers. Oral Oncol. 50, 1000–1004 (2014).
28. McHam, S. A. et al. Who merits a neck dissection after definitive chemoradiotherapy for N2-N3 squamous cell head and neck cancer? Head and Neck 25, 791–798 (2003).
29. Wanebo, H. et al. Surgical Resection Is Necessary To Maximize Tumor Control in Function-Preserving, Aggressive Chemoradiation Protocols for Advanced Squamous Cancer of the Head and Neck (Stage III and IV). Ann. Surg. Oncol. 8, 644–650 (2001).
30. Brizel, D. M. et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 58, 1418–23 (2004).
31. Ranck, M. C. et al. Effect of postradiotherapy neck dissection on nonregional disease sites. JAMA Otolaryngol. - Head Neck Surg. 140, 12–21 (2014).
32. Kim, S. Y. et al. Evaluation of 18F-FDG PET/CT and CT/MRI with histopathologic correlation in patients undergoing salvage surgery for head and neck squamous cell carcinoma. Ann. Surg. Oncol. 18, 2579–84 (2011).
33. Goenka, A. et al. Long-term regional control in the observed neck following definitive chemoradiation for node-positive oropharyngeal squamous cell cancer. Int. J. Cancer 133, 1214–1221 (2013).
34. Hamoir, M. et al. The role of neck dissection in the setting of chemoradiation therapy for head and neck squamous cell carcinoma with advanced neck disease. Oral Oncol. 48, 203–10 (2012).
35. Pellini, R. et al. Planned neck dissection after chemoradiotherapy in advanced oropharyngeal squamous cell cancer: The role of US, MRI and FDG-PET/TC scans to assess residual neck disease. J. Cranio-Maxillofacial Surg. 42, 1834–1839 (2014).
36. Mehanna, H. et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. in New England Journal of Medicine 374, 1444–1454 (Massachussetts Medical Society, 2016).
37. Sherman, E. J. et al. TALK score: Development and validation of a prognostic model for predicting larynx preservation outcome. Laryngoscope122, 1043–1050 (2012).

Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.