Xcytrin Is Well Tolerated in Glioblastoma and Glioma Trials
SAN FRANCISCO-Motexafin gadolinium (Xcytrin), an experimental drug that makes brain tumors more sensitive to radiation, appears to be well tolerated in adults with primary glioblastoma multiforme and children with gliomas, according to preliminary results from two phase I trials presented at the 37th Annual Meeting of the American Society of Clinical Oncology (ASCO).
SAN FRANCISCOMotexafin gadolinium (Xcytrin), an experimental drug that makes brain tumors more sensitive to radiation, appears to be well tolerated in adults with primary glioblastoma multiforme and children with gliomas, according to preliminary results from two phase I trials presented at the 37th Annual Meeting of the American Society of Clinical Oncology (ASCO).
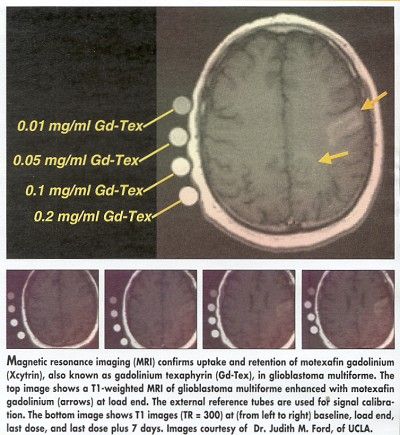
Judith M. Ford, MD, PhD, assistant professor of radiation oncology, UCLA Medical Center, said that median survival for the first 24 patients in the glioblastoma study was 17.3 months (abstract 213). She also reported that imaging studies showed the drug to be present in the tumor throughout the course of treatment, but not in normal brain tissue.
Xcytrin is the first of a new class of porphyrin-like compounds called texaphyrins that are active as reduction oxidation (redox) modulators. The agent enhances radiation-induced apoptosis through caspase activation secondary to futile redox cycling.
Markus Renschler, MD, vice president of oncology clinical development for Xcytrin-developer Pharmacyclics, Inc., Sunnyvale, Calif (www.pcyc.com), said the company is proceeding with a phase II glioblastoma trial in the hope of starting a phase III trial by early next year.
Dr. Renschler told ONI that the 17.3 months median survival in the phase I study of patients with advanced glioblastoma was "provocative," since survival for these patients as reported in the literature is generally only 10 to 11 months.
Pediatric Trial
Response and survival data for the pediatric study of intrinsic pontine (brainstem) gliomas are blocked until completion under the Children’s Oncology Group protocol, said Minesh Mehta, MD, of the University of Wisconsin Medical School, Madison.
He reported (abstract 243) that the trial has established that children can take Xcytrin on a 5-day-a-week dosing schedule. Sixteen children had participated in the dose-escalation trial as of May, with the fourth cohort receiving 1.9 mg/m² five times a week. A fifth cohort at 2.1 mg/m² was expected to start shortly.
Once the maximum tolerated dose in children is established, he predicted the agent would be tried with other childhood tumors as well.
Thus far, Xcytrin’s behavior in children is similar to what has been documented in adults, Dr. Mehta said, and imaging shows the drug is entering the tumor but not normal tissue. He is optimistic that Xcytrin may improve survival for brainstem glioma, a rare but aggressive childhood brain tumor that is usually inoperable and has not responded well to chemotherapy.
"The problem is local. It is not that the disease spreads, so if we can fix the local problem, we have a chance of making an impact," Dr. Mehta told ONI. He anticipates the investigators will be ready for a phase II study by early 2002.
Adult Trials
A phase III trial led by Dr. Mehta is evaluating Xcytrin as an enhancer of whole brain irradiation for patients with brain metastases. This trial recently completed enrollment, and the data are currently being analyzed. Dr.Mehta said that Xcytrin is also being tested in pancreatic cancer.
In the glioblastoma trial reported by Dr. Ford, dose escalation is focused on delivering Xcytrin for a longer course of radiation (6.5 weeks) than was required for whole brain irradiation in the brain metastases trial (10 days). Dr. Ford said that patients tolerated 22 doses of 4 mg/kg without reaching dose-limiting toxicity.
Only four of seven patients were able to complete a 22-dose regimen at 5.3 mg/kg, but she said that the four tolerated the dose so well that she plans to continue investigating at 5 mg/kg.
Drug delivery starts with a 5- or 10-dose, 5-day-a-week loading regimen, and then shifts to three times a week.
The patients can be quite frail, Dr. Ford said. Often, they have had surgery, and many are on steroids, so toxicity and the strain of daily treatment were concerns.
Thus far, Xcytrin has been well tolerated with minor side effects, Dr. Ford said. These include transient green skin and urine in all patients due to drug color, fingertip blisters and cold intolerance in 10 patients, and an allergic rash in 9 patients (8 of whom were on anticonvulsants). Nausea, dyspepsia, and diarrhea were common but mild.
She said that one patient stopped treatment because of a rash and fever, but imaging showed that the drug already administered remained present in the tumor.