XELOX Is Active in Elderly Patients With Metastatic Colorectal Cancer
NAPLES, ITALY-As firstlinetherapy in elderly patients withmetastatic colorectal cancer, XELOX(capecitabine [Xeloda] and oxaliplatin(Eloxatin]) achieved an objectivetumor response rate of 41% (abstract
NAPLES, ITALY-As firstlinetherapy in elderly patients withmetastatic colorectal cancer, XELOX(capecitabine [Xeloda] and oxaliplatin(Eloxatin]) achieved an objectivetumor response rate of 41% (abstract3582). Final results of a phase II multicentertrial conducted by the SouthernItaly Cooperative OncologyGroup, National Tumour Institute inNaples, also showed progression-freesurvival of 8.5 months (95% confidenceinterval [CI]: 6.7-10.3) and overallsurvival of 14.4 months (95% CI:11.9-16.9)."Ongoing phase III trials evaluatingXELOX vs FOLFOX (fluorouracil[5-FU], leucovorin, oxaliplatin) withor without bevacizumab (Avastin) areexpected to confirm that capecitabineshould replace 5-FU/leucovorin as thebackbone of metastatic colorectal cancertherapy," the investigators concluded.Protocol AmendedSafety and efficacy were assessed in76 patients ≥ 70 years, with an EasternCooperative Oncology Group (ECOG)performance status ≤ 2, and life expectancy≤ 3 months. Two XELOXregimens were assessed. The 35 patientsin series 1 received 1,000 mg/m2of oral capecitabine twice daily on days1 to 14 and 85 mg/m2 of oxaliplatin onday 1. In the absence of grade 2 orhigher hematologic toxicity, the doseof oxaliplatin was increased to 100 mg/m2 in cycle 2, and in the absence ofgrade 2 or higher nonhematologictoxicity in cycle 2, the dose of capecitabinewas increased to 1,250 mg/m2twice daily in the third and subsequentcycles.
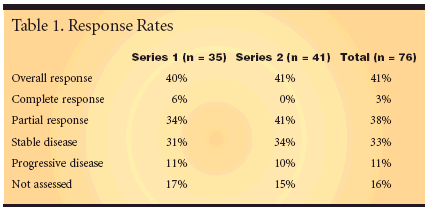
Once 35 patients were treated inthat series, the protocol was amendedfor the next 45 patients: The dose ofoxaliplatin was increased, first to110 mg/m2 and then to 130 mg/m2.After 451 cycles of treatment, witha median of 6 cycles per patient, theoverall response rate was 41% (95%CI, 30%-53%). No differences in activitywere observed between the firstand second series (Table 1).The disease control rate, which includespartial and complete responsesplus stable disease, was 74%.Good Safety ProfileThe investigators reported thatXELOX had a predictable and manageablesafety profile in the elderly,with predominantly mild to moderateadverse events. The second series ofXELOX administration, which hadhigher levels of oxaliplatin but keptcapecitabine at 1,000 mg/m2, producedmore hematologic adverse events.They included grade 3/4 neutropenia,thrombocytopenia, and anemia, whichdid not occur in the first series. Therewere no treatment-related deaths."XELOX also offers benefit to thepatient in terms of convenience, reduceddiscomfort, and avoidance ofcentral venous access vs infusional5-FU/leucovorin regimens," the investigatorsconcluded. "These benefitsare particularly important in elderlypatients, who may have moredifficulty in traveling to/from the oncologyclinic."