Your Patient With Melanoma: Staging, Prognosis, and Treatment
Melanoma affects persons of all ages, causing more years of lost life than any other cancer except leukemia.[1] The American Cancer Society estimates that about 68,720 new melanomas will be diagnosed in the US in 2009, with more than 8,650 deaths, and an estimated lifetime risk of 1 in 50 for whites, 1 in 200 for Hispanics, and 1 in 1,000 for blacks.[2]
ABSTRACT: Melanoma, a cancer of melanocytes, pigment-producing cells in the skin, is the most serious form of skin cancer. Its incidence is increasing rapidly and reaching epidemic proportions. When detected early, it is considered curable, but when detected at later stages it is arguably one of the most lethal malignancies and is the cause of more years of lost life than any other cancer except leukemia. Because most cutaneous melanomas are visible, however, melanoma in general is a cancer highly amenable to early detection. Surgery is standard treatment for localized melanoma. There is no standard therapy fo advanced-stage melanoma. Metastatic melanoma disseminates widely and it frequently involves sites that are not commonly affected in other cancers, such as the gastrointestinal tract and skin. The median survival time for patients with metastatic melanoma is less than 1 year. Despite these grim statistics, long-term survival occurs occasionally. This article will review diagnosis, staging, and treatment for malignant melanoma and will discuss the nursing role in the care of patients with melanoma.
Melanoma affects persons of all ages, causing more years of lost life than any other cancer except leukemia.[1] The American Cancer Society estimates that about 68,720 new melanomas will be diagnosed in the US in 2009, with more than 8,650 deaths, and an estimated lifetime risk of 1 in 50 for whites, 1 in 200 for Hispanics, and 1 in 1,000 for blacks.[2] Melanoma is currently the second leading cancer diagnosis among women under 40 years of age, and the third leading cancer diagnosis among men in this age group.[3] Five-year survival has increased from 80% in the period from 1975–1976 to 92% between 1995 and 2001.[4] The improvement in survival, however, is limited to younger patients.
Among those over the age of 54 years, both the incidence of melanoma and the mortality rate are increasing, with the highest mortality occurring in older white males. Globally, melanoma incidence varies widely, with annual age-adjusted incidence rates of < 1 per 100,000 persons in Japan and India, to 37.8 and 29.4 per 100,000 among Australian males and females, respectively. African Americans have the lowest risk of melanoma, though they tend to present with metastatic disease more frequently.[5]
Risk factors
TABLE 1

Risk Factors for Melanoma
Certain populations have been identified as being at higher risk than the average person for development of melanoma (see Table 1). They include persons with lighter complexions, an inability to tan, blonde or red hair and blue eyes, and those with multiple nevi, particularly atypical nevi. Immunosuppression, sun sensitivity, and exposure to ultraviolet radiation are additional risk factors.[6] Patients with multiple nevi have a relative risk of 5–12 of developing melanoma, and those with multiple atypical nevi have a relative risk of 7–27.[7] Patients at the greatest risk for developing melanoma include those with a strong family history of melanoma and multiple clinically atypical moles. Interestingly, though some moles are precursors to melanoma, dysplastic nevi are considered to be cutaneous markers that identify persons at an increased risk for developing melanoma compared with the general population. Another higher-risk population includes patients with a personal history of melanoma, with the greatest risk period being 2 years after diagnosis.[8]
Genetics
The greatest environmental risk factor for development of melanoma is sun exposure; however, genetics also has an impact on melanoma risk. In the past decade, progress has been made towardidentifying genes that contribute to inherited susceptibilityto melanoma.[9] Among themultiple genes thatconfera higher predisposition to melanoma is cyclin-dependent kinase inhibitor A (CDKN2A), also known as p16. This gene is located on chromosome 9 andregulates the cell cycle.
Germ line mutations in this gene have been identified in about 20% of melanoma-prone families that have been studied to date. It is estimated that people with a CDKN2A alteration havea greater than 50% chance of developing melanoma during their lifetime as well as an increased risk of developing more than one primary melanoma, andthey are typically diagnosed at younger ages.Furthermore, persons with CDKN2Amutations have demonstrated a 13–22 fold increase in development of pancreatic cancer,[10] and an increased risk of developing breast cancer.[11] Though only a small proportion of people with melanoma or a family history of melanoma have hereditary alterations in CDKN2A, understanding these increased risks is paramount to management of this population.
While assays for germ line CDKN2A mutationsare commercially available, the Melanoma Genetics Consortium argues that clinical CDKN2A genetic testing is premature, owing to uncertainties regarding penetrance and the efficacy of melanoma prevention and risk-reduction strategies.Providers caring for patients with melanoma who may have undergone genetic testing must recognize that negative CDKN2A test results in families without known mutations would not disprove hereditary melanoma, as certain mutations may be undetectable with current molecular methodologies or because mutations might be present in other melanoma suppressor genes.[12] Providers must also anticipatethat somepatientsmaynot wish to know their status, sincepositive results may increase anxiety.On the other hand, negative results may contribute to a false sense of security.Genetic counseling should be considered to address these concerns.
Diagnosis
What Does Melanoma Look Like?
FIGURE 1
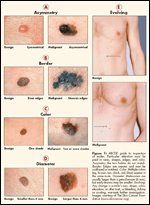
ABCDE guide to inspection of moles. Particular attention must be paid to sizes, shapes, edges, and color. Assymetry: the two halves do not match. Border: Edges are uneven and may be scalloped or notched. Color: Multiple colors (eg, brown, tan, black, red, blue) appear in the same mole. Diameter: Melanomas are usually larger than a pencil eraser (6 mm), but early lesions may be smaller. Evolving: Any change in a mole’s size, shape, color, elevation, or other trait, or bleeding, itching or crusting, warrants further investigation. Images courtesy of The Skin Cancer Foundation (www.skincancer.org).
Pigmented lesions can be difficult to evaluate even for very experienced clinicians, particularly in patients with multiple nevi. When evaluating atypical nevi, it is essential to be mindful of the goal of screening: to properly identify melanoma in its earliest stages, when it is considered curable. It is standard practice to remove any pigmented or nonpigmented lesion that is suggestive of melanoma and to perform histologic examination.[13,14] This is necessary to ensure that the lesion is not a melanoma, or if it is, that the proper recommendations for further treatment are determined. The implications for missing a melanoma are far too great to forego biopsy of moderately or highly suspicious lesions. Identifying such lesions accurately is a challenge, however.
It is important to be cognizant that the majority of melanomas arise de novo rather than from pre-existing nevi.[15] Only about one-third of melanomas follow the ABCDE rule of melanoma recognition (see Figure 1), and many lesions determined to be melanoma are clinically innocuous. Argenziano et al.[13] emphasize that while the majority of melanomas exhibit a sufficient array of clinical features to justify biopsy, melanoma may occasionally mimic a variety of benign lesions, such as common melanocytic nevi or seborrheic keratosis.
Furthermore, very early melanomas and nodular melanoma can sometimes be symmetric, well-demarcated, homogenously pigmented, and less than 5 mm in size.[16] Amelanotic melanoma can mimic other skin lesions, particularly more benign lesions, therefore it must be considered in the differential diagnosis of nonpigmented lesions. Though melanomas frequently display irregularities in shape, color, and border, these features are neither invariant nor specific.[8]
Clinicians use several general approaches to recognize melanoma. These include overall pattern recognition (gestalt; eg, instant recognition of an image) as well as analytical recognition criteria such as the ABCDEs of melanoma. Differential recognition, also known as the “ugly duckling,” is based on the concept that nevi in the same individual tend to resemble one another and that melanoma often deviates from the individual’s nevus pattern.[14]
Staging and Prognostic Factors
The American Joint Committee on Cancer staging system for cutaneous melanoma was revised in 2002 based on an analysis of prognostic factors in 17,600 cases.[3] (The 7th Edition of the AJCC Cancer Staging Manual will be published in August 2009.) Melanomas without regional lymphatic spread are designated as Stage I or II. Staging of node-negative melanomas is based on the thickness of the primary lesion; ulceration (absence of an intact epidermal layer overlying the melanoma); and, for thin melanomas (< 1 mm), the anatomic depth of invasion (see Tables 2 and 3).
TABLE 2
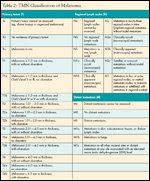
TNM Classification of Melanoma
Stage III is defined by involvement of regionallymphaticsorlymph nodes. The tumor burden in the lymph nodes ishighly predictive of outcome. It is represented by the number of nodes involved, and whether involvement is microscopic (clinically unapparent prior to surgery), or macroscopic (clinically apparent).
Stage IV melanoma represents distant metastatic spread, and is stratified according to sites of involvement and lactate dehydrogenase (LDH) level.Though the median survival time is less than 1 year, there is a meaningful proportion of long-term survivors (15.7%) among patients with metastases limited to skin and subcutaneous tissues (M1a). Based on these T, N, and M criteria, patients can be grouped according to prognosis(see Tables 2 and 3). Regardless of stage,age and male gender are adverse prognostic factors, and melanomas arising on the head and neck are associated with a worse prognosis than those at other sites.
Treatment of Melanoma
Surgery
TABLE 3

Stage Groupings for Cutaneous Melanoma
Wide local excision is the treatment for primary melanomas. Surgical margins are determined based on the thickness of the lesions, with margin guidelines supported by several large, randomized clinical trials and recommended by the National Comprehensive Cancer Network (NCCN). Patient outcomes demonstrate that for thin, primary melanomas (of 1.0 mm or less), a 1-cm margin is adequate. For lesions thicker than 1 mm, 2-cm margins are recommended (see Table 4).Margins may be modified to accommodate individual anatomic or cosmetic considerations.Evaluation for regional nodal involvement is a critical part of staging, since nodalstatus is the single most important prognostic factor in melanoma.
For patients with no clinical evidence of nodal involvement, elective lymph node dissection does not improve survival and hasbeen superseded by sentinel lymph node assessment.Sentinel lymph nodemapping involves injection of aradiotracer or dye around the primary melanoma.The tracer istracked to the “sentinel” node or nodes, which can be excised through a small incision.Sentinel node biopsy is areliable means of identifying melanoma in regional lymph nodes.
If the sentinel nodes do not contain evidence of metastatic melanoma, then the remaining lymph nodes in the basin are also highly likely to be free of disease and thus further nodal dissection is spared.Completion (or therapeutic) nodal dissection is advised when a positive sentinel node has been identified.Sentinel node evaluationshould be considered for patients with melanomas > 1 mm, or in the case of lesions less than 1 mm, if there are adversepathologic factors such as ulcerationormitoses.
High-Risk Melanoma
TABLE 4
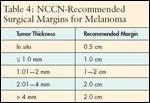
NCCN-Recommended Surgical Margins for Melanoma
The definition of high riskisambiguous but generally refers to patients with a predicted likelihood of distant failure of more than 50% after stage-appropriate surgery.[17] Prognosis for patients with cutaneous melanoma is dependent on the number of involved regional nodes and the thickness of the primary tumor. Patients with pathologic or clinical evidence of regional nodal metastases and those with thick primary lesions (T4) have been demonstrated to be at high risk of disease recurrence.[18] Once palpable nodal disease develops, the ability to provide effective local/regional control and long-term survival is diminished.[19]
Standard treatment typically includes resection of the primary tumor with appropriate margins, and in the case of patients with palpable lymphadenopathy, therapeutic dissection of the regional nodal basin.Imagingstudies such as PET or CT scansare not indicated for asymptomatic patients since detection of distant metastases is uncommon.[20]
Adjuvant Therapy
High-dose interferon (HDI) alfa-2b (Intron A) is the only US Food and Drug Administration (FDA)-approved adjuvant therapy for resected, high-risk melanoma, and it remains the best-evaluated and most effective available option aimed at reducing the risk of relapse in patients with high-risk melanoma. Its efficacy remains controversial, however. The role of interferon alfa-2b has been established by three large clinical trials conducted in the US, all demonstrating an improvement in relapse-free survival (RFS), and two showing an improvement in overall survival (OS).
HDI seems to reduce the risk of relapse in only a subgroup of patients,[3,21] and because the medication is expensive and toxicity associated with therapy can be significant, acceptance of the treatment has been limited.[21] Nevertheless, the toxicities appear justified by the potential benefit.[22] Kirkwood et al. [23] recognize that treatment is indeed associated with significant toxicity, but they argue that the majority of patients can tolerate the 1 year of therapy required if clinicians provide education about the treatment and its anticipated toxicities in conjunction with appropriate dose modifications and available supportive care.
Metastatic Disease
No treatment has been demonstrated to improve median survival in metastatic melanoma, and response rates to systemic therapies are low. Despite these grim statistics, there are occasional long-term survivors, including up to 15% of patients with M1a metastases.[3] A small and highly select group of patients with advanced melanoma may achieve prolonged freedom from relapse after surgical resection of metastases.
Chemotherapy. Melanoma is relatively refractory to standard cytotoxic agents. Objective response rates to single-agent chemotherapy are in the range of 10%–23%, and are typically of brief duration.[3] Response rates to combination chemotherapy are somewhat higher, but toxicity is increased with the use of multiple agents and no survival advantage has been demonstrated. Dacarbazine (DTIC) has been considered a “standard” treatment, yet responses are observed in less than 20% of patients. In a randomized trial, DTIC was compared with the oral methylating agent temozolomide (Temodar) and response rates were similar (13.5% with temozolomide and 12.1% with DTIC). Progression-free survival was slightly prolonged in the temozolomide arm (1.9 vs 1.5 months), but there was no statistical difference in survival (7.7 and 6.4 months with temozolomide vs DTIC, respectively).[24]
Immunotherapy. Interleukin-2 (IL-2) is a central regulator of the cellular immune response, inducing activation and proliferation of T cells and NK cells. In vitro, it stimulates development of lymphokine- activated killer (LAK) cells, which can lyse autologous tumor cells. IL-2 treatment has been evaluated extensively in patients with metastatic melanoma and was approved by the FDA in 1998. Initial studies with HD bolus IL-2 demonstrated overall response rates in 15%–20% of patients, with 4%–6% of patients achieving durable responses.[25] Patients with metastases limited to skin and subcutaneous sites appear most likely to benefit.[3]
Long-term follow-up of patients treated in early HD IL-2 trials confirms that IL-2 produces a significant clinical benefit for a minority of patients. The substantial toxicity associated with the agent, including a capillary leak syndrome, hemodynamic instability, and a high risk of infection make its use restricted to patients with excellent organ function and performance status, and to experienced clinicians in institutions capable of providing a comprehensive and intensive level of care. IL-2 has been evaluated in various contexts: with adoptive cellular immunotherapy using autologous LAK cells or tumor-infiltrating lymphocytes, with other cytokines, and with chemotherapy agents and vaccines. To date, none of these approaches has demonstrated clear superiority compared with IL-2 given as a single agent.
Recent investigations in immunotherapy have focused on overcoming barriers to generating a sustained and effective antitumor immune response.[3] Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) is expressed on activated T cells. When CTLA4 is engaged by its ligands on antigen-presenting cells, it sends a negative signal to turn off the T cell. Ipilimumab, a monoclonal antibody that binds to CTLA4 and blocks it from binding to the antigen-presenting cell, is currently in clinical trials, and has been demonstrated to enhance antitumor immune responses by removing the negative immune signal.[26] Complete responses and sustained partial responses have been reported in up to 13% of patients with metastatic melanoma treated with these agents, but serious autoimmune toxicity, including enterocolitis and hyphophysitis, has also been observed.[26]
As with HDI in the adjuvant setting, there appears to be a correlation between autoimmune phenomena, such as colitis, diarrhea, vitiligo, dermatitis, pruritis, thyroiditis, and panhypopituitarism and the efficacy of treatment. Colitis induced by these agents responds to treatment with corticosteroids and other medications, but early recognition of symptoms and institution of treatment is essential, making patient education crucial. The importance and rationale of early reporting of symptoms, particularly loose stools, must be stressed to patients.
Targeted Therapy for Melanoma
Over the past several years, important advances in oncology have resulted from a detailed understanding of the molecular biology of cancer and the subsequent development of targeted drugs that inhibit key pathways on which the growth and survival of a particular cancer depend.[26] Research has begun to uncover the genetic abnormalities underlying dysregulated growth, resistance to apoptosis, invasion, and metastasis in melanoma. Substantial molecular heterogeneity has been discovered among melanomas, but a number of mutations occur with high frequency and appear to be critical for survival and growth. Activating mutations in the mitogen-activated protein (MAP) kinase signal transduction pathway are found in the majority of melanomas, most frequently involving the B-raf and N-ras genes.[27]
Inhibitors of B-raf and MEK. Approximately 50%–70% of melanomas harbor mutations in the signaling protein B-raf. In vitro and in vivo data suggest that mutated B-raf plays a key role in the pathogenesis of melanoma.[27] The oral kinase inhibitor sorafenib (Nexavar), approved for treatment of advanced renal cell carcinoma and unresectable hepatocellular carcinoma, was found to be ineffective in phase III trials in melanoma. However, more potent and selective B-raf inhibitors are currently being evaluated in early-phase trials. Preclinical studies have suggested that cancers with B-raf mutations are exquisitely sensitive to inhibition of MAP extracellular signal–regulated kinase (MEK), the signaling molecule immediately downstream from B-raf in the MAP kinase cascade.[28] Specific MEK inhibitors are therefore also of great interest and are currently in phase I and II trials.
C-kit in melanoma. A small subgroup of melanomas harbor mutations or amplification in the gene encoding the receptor tyrosine kinase c-kit. These include some melanomas arising on mucosal surfaces (anorectal, vaginal, sinonasal, etc.), as well as acral melanomas (those arising on the hands and feet) and some melanomas arising in patients with a history of chronic sun-damaged skin. These melanomas have generated intense interest because of the availability of drugs that potently inhibit c-kit, including imatinib (Gleevec), dasatinib (Sprycel), and nilotinib (Tasigna). These agents are highly effective in gastrointestinal stromal tumors that are also characterized by c-kit mutations. Preliminary reports suggest that these agents can induce dramatic and sustained responses in patients with melanomas harboring c-kit mutations, the first instance of effective targeted therapy for melanoma. Several clinical trials are ongoing.
Nursing Management
The role of the oncology nurse in the care of patients with melanoma is essential. Owing to the lack of effective systemic treatments, patients frequently are treated in large academic referral centers, and therefore many nurses will have limited yet important interactions with melanoma patients. Nurses can help to educate patients about an often complicated disease, offering psychological support and guidance as well as reiterating goals of care and the rationale for specific treatment recommendations.
Oncology nurses who care for patients with melanoma must understand the unique toxicity profiles of many of the novel therapies and anticipate their side effects. Patient education and counseling regarding prompt reporting of developing symptoms is crucial to management. For other agents, such as IL-2, which has a tremendous toxicity profile, nurses require specialty training and are a cornerstone in the delivery of this therapy.
Regardless of experience with patients who have melanoma, oncology nurses must individualize support and counsel according to the potential trajectory of the patient’s melanoma experience. Of critical importance is the nursing role in primary prevention strategies such as early detection, use and proper application of sunscreening products, skin self-examination, and access to care for a suspicious lesion.
Financial Disclosure:Krista M. Rubin serves on a speakers bureau for Schering Plough Oncology and Novartis and on an advisory board of Schering Plough Oncology. Donald P. Lawrence serves on an advisory board of Schering Plough Oncology.
References:
1. High W: Malpractice in dermatopathology: Principles, risk mitigation, and opportunities for improved care for the histologic diagnosis of melanoma and pigmented lesions. Clin Lab Med 28(2):261â284, 2008. 2. American Cancer Society: What Are the Key Statistics About Melanoma? Last revised May 14, 2009. Available at: http://www.cancer.org/docroot/cri/content/cri_2_4_1x_what_are_the_key_statistics_for_melanoma_50.aspaboutabout. Accessed on May 26, 2009.3. Lawrence D, Rubin K: Melanoma, in Chabner BA, Lynch TJ, Longo DL (eds): Harrison’s Manual of Oncology. New York, NY, McGraw Hill, 2008.4. Ries L, Eisner MP, Kosary CL, et al (eds): SEER Cancer Statistics Review, 1975â2002. Bethesda, MD, National Cancer Institute, 2005.5. GLOBOCAN 2002 Database. Available at: http://www-dep.iarc.fr/GLOBOCAN_frame.htm. Lyon, France, International Agency for Research on Cancer, 2002. 6. Miller AJ, Mihm MC Jr: Melanoma. N Engl J Med 355(1):51â65, 2006.7. Risser J, Pressley Z, Veledar E, et al: The impact of total body photography on biopsy rate in patients from a pigmented lesion clinic. J Am Acad Dermatol 57(3):428â434, 2007.8. Tsao H, Atkins MB, Sober AJ: Management of cutaneous melanoma. New validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 19:3622 â3634, 2004.9. Tsao H, Carpiniello LM: Inherited susceptibility to melanoma. Available [with UpToDate subscription] at: http://www.uptodate.com/patients/content/topic.do?topicKey=~L2M/td7TVdnDu2&selectedTitle=1~150&source=search_result. Accessed on May 29, 2009.10. Rieder H, Bartsch DK: Familial pancreatic cancer. Fam Cancer 3(1):69â74, 2004.11. Niendorf KB, Tsao H: Cutaneous melanoma: Family screening and genetic testing. Dermatol Ther 19(1):1â8, 2006.12. Fowler ES, Wolfe KS, McChesney T, et al: Genetic testing for hereditary melanoma: Controversial, standard of care, or somewhere between the two? Community Oncol 3(3):158â161, 2006. 13. Argenziano G, Zalaudek I, Ferrara G, et al: Dermoscopy features of melanoma incognito: Indications for biopsy. J Am Acad Dermatol 56(3),508â513, 2007.14. Scope A, Dusza S, Halpern A, et al: The “ugly duckling” sign: Agreement between observers. Arch Dermatol 144(1):58â64, 2008.15. Fuller SR, Bowen GM, Tanner B, et al: Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg 33(10):1198â1206, 2007. 16. Puig S, Argenziano G, Zalaudek I, et al: Melanomas that failed dermoscopic detection: A combined clinicodermoscopic approach for not missing melanoma. Dermatol Surg 33(10):1262â1273, 2007.17. Kirkwood JM, Tarhini AA: Adjuvant high-dose interferon-alpha therapy for high-risk melanoma. Forum (Genova) 13(2):127â140, 2003.18. Kirkwood J, Ibrahim J, Sonda V, et al: High- and low-dose interferon alfa-2b in high-risk melanoma: First analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol 18(12):2444â2458, 2000.19. Essner R, Kaushal A, Flaherty K: Melanoma and other skin cancers, in Pazdur R, Wagman LD, Camphausen KA, et al (eds): Cancer Management: A Multidisciplinary Approach, 11th ed. Manhasset, NY, CMP Medica, 2008.20. Buzaid AC, Gershenwald JE, Ross MI: Initial staging investigations for melanoma, in Thompson JF, Morton DL, Kroon BBR (eds): Textbook of Melanoma. London, England, Martin Dunitz, Ltd., Taylor & Francis Group, 2004.21. Gogas H, Ioannovich J, Dafni U, et al: Prognostic significance of autoimmunity during treatment for melanoma. N Engl J Med 354(7):709â718, 2006. 22. Sabel MS, Sondak VK: Point: Interferon-alfa for adjuvant therapy for melanoma patients. J Natl Compr Canc Netw 2:61, 2004. 23. Kirkwood JM, Bender C, Agarwala S, et al: Mechanisms and management of toxicities associated with high-dose interferon alfa-2b therapy. J Clin Oncol 20(17):3703â3718, 2002.24. Balch C, Buzaid A, Soong S, et al: Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 19(16):3635 â3648, 2001. 25. Atkins M, Sosman J: Cytotoxic chemotherapy and biochemotherapy for metastatic melanoma. 2007. Available online at: http://www.uptodate.com/patients/content/topic.do?topicKey=~L2M/td7TVdnDu2&selectedTitle=1~150&source=search_result. Accessed on May 26, 2009.26. Rubin K: Management of metastatic melanoma: Nursing challenges today and tomorrow. Clin J Oncol Nurs 3(1):81â89, 2009. 27. Chudnovsky Y, Khavari PA, Adams AE: Melanoma genetics and the development of rational therapeutics. J Clin Invest 115(4):813â824, 2005.28. Solit DB, Santos E, Pratilas CA, et al: 3’-deoxy- 3’-[18F] fluorothymidine positron emission tomography is a sensitive method for imaging the response of BRAF-dependent tumors to MEK inhibition. Cancer Res 67(23):11463â11469, 2007.29. Greene FL, Page DL, Fleming ID, et al (eds): AJCC Cancer Staging Manual, 6th ed. New York, NY, Springer-Verlag, 2002.