3 Things You Should Know About the Multimodal Treatment of SCLC
Here are 3 things you should know about the multimodal treatment of patients with SCLC.
Meet the experts

Chemotherapy and radiotherapy have long-standing roles in the treatment of patients with small-cell lung cancer (SCLC). Refinements continue to be made in the delivery of radiotherapy, and the role of immunotherapy (IO) continues to evolve as an integral component of SCLC treatment. Here are 3 things you should know about the multimodal treatment of patients with SCLC.
1 Consolidation IO provides a benefit to chemoradiotherapy in limited stage-SCLC treatment.
Concurrent chemoradiotherapy (CRT) using combination platinum plus etoposide therapy has been the standard of care in limited stage-SCLC (LS-SCLC) for 3 decades after a meta-analysis of 13 trials demonstrated a 5.4% overall survival (OS) improvement with CRT compared with chemotherapy alone.1 The phase 3, placebo-controlled ADRIATIC trial (NCT03703297) assessed whether immune checkpoint inhibitor (ICI) therapy after CRT could improve outcomes in patients with LS-SCLC.2 A total of 730 patients received either durvalumab, durvalumab plus tremelimumab, or placebo following CRT. The median OS with durvalumab was 55.9 months (95% CI, 37.3-not reached) vs 33.4 months (95% CI, 25.5-39.9) with placebo (HR, 0.73; 98.321% CI, 0.54-0.98; P = .01). The median progression-free survival (PFS) with durvalumab was 16.6 months (95% CI, 10.2-28.2) vs 9.2 months (95% CI, 7.4-12.9) with placebo (HR, 0.76; 97.195% CI, 0.59-0.98; P = .02). Select adverse events (AEs) are shown in Figure 1.2
FIGURE 1. Adverse Events Reported in the ADRIATIC Trial
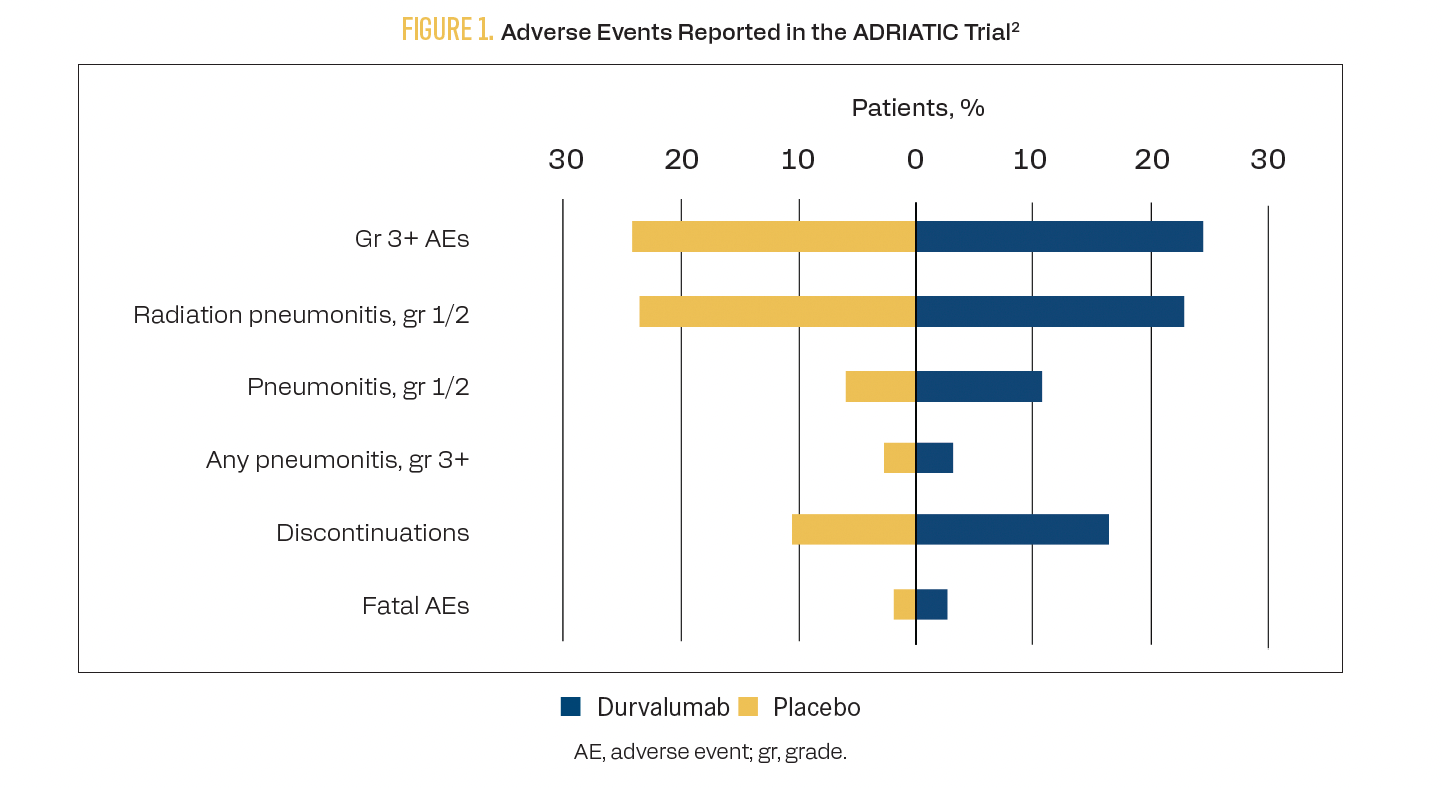
Other IO regimens have been tested in combination with CRT with less-positive results. In the phase 3 NRG LU005 study (NCT03811002), concurrent and consolidation atezolizumab did not provide a benefit to OS, PFS, distant metastasis-free survival, or local failure rates compared with CRT alone.3 The phase 3 KEYLYNK-013 study (NCT04624204) likewise will examine whether the addition of pembrolizumab to concurrent CRT and consolidation therapy provides a survival benefit.4 Results are forthcoming.
2 Best practices for delivering radiotherapy during CRT in patients with LS-SCLC continue to evolve.
Several studies have compared once-daily with twice-daily administration of radiotherapy. The Intergroup 0096 trial demonstrated 5-year survival rates of 26% with twice-daily treatment vs 16% with once-daily treatment (P = .04).5 However, patients receiving twice-daily treatment experienced twice the risk of grade 3 or greater esophagitis (32% vs 16% with once daily; P < .001).
The phase 3 CONVERT superiority study (NCT00433563) also addressed the question of twice-daily vs hypofractionated daily treatment.6 A total of 547 patients received cisplatin plus etoposide chemotherapy and were randomly assigned to receive either 45 Gy delivered in 30 fractions twice daily or 66 Gy delivered in 33 fractions daily. There were no statistically significant differences in OS or PFS between the 2 groups, thus demonstrating that hypofractionated daily treatment is not superior to twice-daily treatment. Between the 2 groups, there were similar rates of grade 3 or 4 esophagitis (19% in each) and radiation pneumonitis (3% with twice-daily treatment vs 2% with once-daily treatment).
Both once-daily and twice-daily treatments are accepted practices for patients with LS-SCLC.7 A survey of radiation oncologists in the US demonstrated that twice-daily treatment is only administered in 24% of cases, despite 40% of radiologists preferring twice-daily treatment.8 Reasoning behind the clinical preference for once-daily treatment includes convenience for patients and improved logistics for clinic schedules.
The role of prophylactic cranial irradiation (PCI) in the treatment of patients with SCLC has also evolved. Historically, PCI was thought to decrease the risk of metastasis to the brain; however, it came with an increased risk of neurological toxicity. A meta-analysis of 987 patients with SCLC demonstrated a 5.4% increase in 3-year OS with PCI vs without (HR, 0.84; 95% CI, 0.73-0.97; P = .01).9 However, brain imaging was not required of study participants prior to treatment, so it is unknown whether PCI prevented metastases or simply treated them. A later trial that did conduct MRI scans on all participants and only enrolled patients with extensive-stage SCLC (ES-SCLC) who had any response to initial chemotherapy and no brain metastases demonstrated no OS benefit with PCI (HR, 1.27; 95% CI, 0.96-1.68; P = .094).10 These results have led to a decrease in the use of PCI in this patient population.
3 Recent advancements in biomarker-driven approaches pave the way for individualized treatment in SCLC.
SCLC can be grouped into 4 subtypes (A, N, P, I) based on differential expression of transcription factors ASCL1, NEUROD1, POU2F3, or none of these but with an inflamed gene signature that includes higher expression of immune checkpoints and human leukocyte antigens (Figure 2).11
FIGURE 2. Subtype Distribution in SCLC
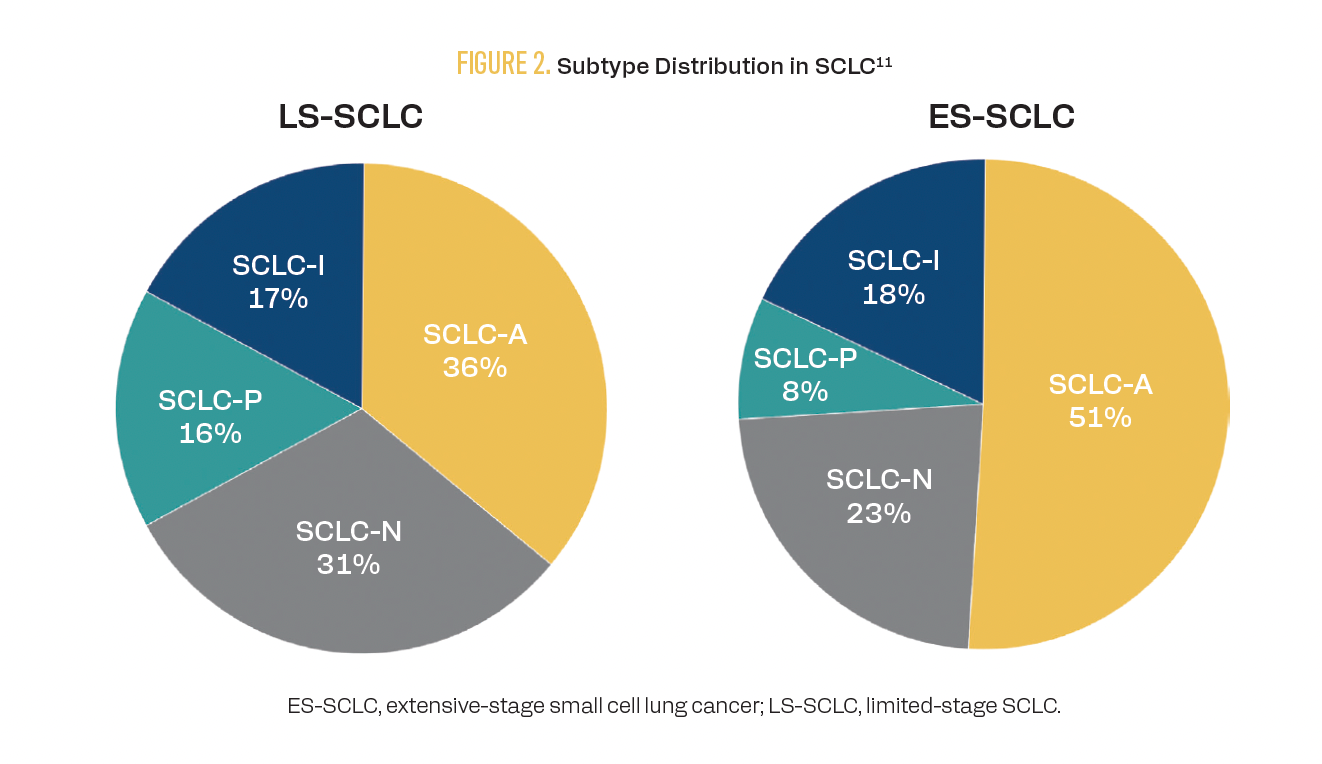
Results from some studies have suggested that the addition of ICIs to standard chemotherapy regimens may provide an increased benefit to patients with the ES-SCLC-I subtype. The phase 3 IMpower133 trial (NCT02763579) demonstrated an OS of 12.3 months across subtypes when atezolizumab was added to chemotherapy vs 10.3 months when placebo was added to chemotherapy (HR, 0.76; 95% CI, 0.60-0.95; descriptive P = .0154).12 Although the study was not powered to assess individual subtypes, subsequent analysis suggested an even greater improvement in OS in patients with the SCLC-I subtype treated with atezolizumab (18 months vs 10 months with placebo; HR, 0.566; 95% CI, 0.321-0.998).11
Likewise, patients with the ES-SCLC-I subtype benefited the most from ICI therapy in the phase 3 CASPIAN trial (NCT03043872).13 In this study, 805 treatment-naive patients with ES-SCLC were treated with etoposide-platinum chemotherapy and either durvalumab or durvalumab plus tremelimumab. A total of 182 patients underwent transcriptional molecular subtyping. An OS benefit was demonstrated with durvalumab treatment vs chemotherapy alone across subtypes (HR, 0.81; 95% CI, 0.67-0.97), and the OS was numerically highest in the 10 patients with the SCLC-I subtype (median, 24.0 months).
Future studies matching potential treatments to patients’ subtypes will determine the course of individualized therapy in SCLC.
Key References
2. Cheng Y, Spigel DR, Cho BC, et al; ADRIATIC Investigators. Durvalumab after chemoradiotherapy in limited-stage small-cell lung cancer. N Engl J Med. 2024;391(14):1313-1327. doi:10.1056/NEJMoa2404873
8. Farrell MJ, Yahya JB, Degnin C, et al. Radiation dose and fractionation for limited-stage small-cell lung cancer: survey of US radiation oncologists on practice patterns. Clin Lung Cancer. 2019;20(1):13-19. doi:10.1016/j.cllc.2018.08.015
11. Gay CM, Stewart CA, Park EM, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 2021;39(3):346-360.e7. doi:10.1016/j.ccell.2020.12.014
For Full Reference list, visit
https://www.gotoper.com/ttlc25sclc-postref
RELEASE DATE:May 1, 2025
EXPIRATION DATE:May 1, 2026
LEARNING OBJECTIVES
Upon successful completion of this activity, you should be better prepared to:
•Evaluate current standards of care for limited-stage small cell lung cancer (LS-SCLC), including the role of concurrent chemoradiotherapy and the integration of evidence-based therapeutic strategies.
•Interpret emerging clinical trial data to assess the potential impact of immunotherapy and combination approaches in LS-SCLC management.
•Discuss the practical application of immunotherapy in LS-SCLC, including patient selection, timing, duration, and strategies to monitor and manage treatment-related toxicities.
Accreditation/Credit Designation
Physicians’ Education Resource®, LLC, is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Physicians’ Education Resource®, LLC, designates this enduring material for a maximum of 0.25 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Acknowledgment of commercial support
This activity is supported by an educational grant from AstraZeneca Pharmaceuticals.
Off-label disclosure/disclaimer
This activity may or may not discuss investigational, unapproved, or off-label use of drugs. Learners are advised to consult prescribing information for any products discussed. The information provided in this activity is for accredited continuing education purposes only and is not meant to substitute for the independent clinical judgment of a health care professional relative to diagnostic, treatment, or management options for a specific patient’s medical condition. The opinions expressed in the content are solely those of the individual faculty members, and do not reflect those of PER or any company that provided commercial support for this activity.
Instructions for participation/how to receive credit
1. Read this activity in its entirety.
2. Go to https://www.gotoper.com/ttlc25sclc-postref to access and complete
the posttest.
3. Answer the evaluation questions.
4. Request credit using the drop-down menu.
YOU MAY IMMEDIATELY DOWNLOAD YOUR CERTIFICATE.
