Utility of the Diagnosis-Specific Graded Prognostic Assessment for Prognostication in Leptomeningeal Disease
This retrospective study included 64 patients with LMD, with primary cancers represented in the diagnosis-specific Graded Prognostic Assessment (DS-GPA) at a single institution over 5 years.
Abstract
Leptomeningeal disease (LMD) is the spread of cancer cells to the arachnoid mater, pia mater, and cerebrospinal fluid. It occurs in 5% to 10% of solid organ cancers, with higher rates in breast, lung, and melanoma cancers. The prognosis for patients with LMD remains poor, with a median survival of 1.5 months without treatment and 2 to 3 months with treatment, despite advances in cancer treatment.
This retrospective study included 64 patients with LMD with primary cancers represented in the diagnosis-specific Graded Prognostic Assessment (DS-GPA) at a single institution over 5 years. Patient characteristics, treatment, and overall survival (OS) data were collected. Statistical analyses included descriptive statistics, log-rank tests, and Cox proportional hazards regression models.
The median OS for the 64 patients with LMD was 2.6 months, with no statistically significant differences among cancer types. Though not statistically significant, those with higher DS-GPA scores trended toward longer survival in breast and lung cancer cohorts. Patients with LMD on imaging confined to 1 location (cerebrum, cerebellum, spine, or cranial nerves) and receiving systemic chemotherapy alone also had longer survival.
The DS-GPA tool is promising for LMD prognostication and may be strengthened by incorporating imaging and chemotherapy characteristics. Larger, multicenter studies are needed to validate its prognostic utility.
Keywords: Leptomeningeal disease, diagnosis-specific graded prognostic assessment, prognosis, overall survival, breast cancer, lung cancer.
Introduction
Leptomeningeal disease (LMD) is the spread of cancer cells to the pachymeninges (ie, arachnoid mater, pia mater, and cerebrospinal fluid [CSF]).1 The incidence of LMD in solid organ cancers is likely underestimated but reported to be 5% to 10%.2 Breast cancer (12%-35%), lung cancer (10%-26%), melanoma (5%-25%), gastrointestinal cancers (4%-14%), and cancers of unknown primary (1%-7%) are the common cancers associated with LMD.2 Advances in cancer diagnosis and treatment have led to earlier detection and increased survival, which has resulted in higher incidence and prevalence of LMD. Symptoms of LMD vary based on the area of involvement in the craniospinal axis but commonly include headache, neck or back pain with radicular radiation, weakness, seizures, and mental status changes.3 Diagnosis of LMD is typically made with MRI and/or CSF analysis.
Despite advances in cancer treatment, LMD still carries a poor prognosis, with a median survival of 1.5 months without treatment and a median survival of 2 to 3 months with treatment.2 Study investigators have identified several factors affecting survival in LMD in univariate analyses, but only a few were significant in the multivariate analyses, with varying factors identified across studies. Some independent prognostic factors associated with improved survival were female sex, longer duration of neurological symptoms at the time of presentation, longer time from diagnosis of primary tumor to diagnosis of LMD, and receipt of systemic chemotherapy.4-6 Conversely, factors associated with worse survival included poor performance status (ECOG status of 2-4 or Karnofsky performance score [KPS] score of < 60), MRI-confirmed/gross LMD, elevated CSF protein level (≥ 112 mg/dL), and clinical involvement of cerebral leptomeninges.5,7 Prognostication at diagnosis is crucial in deciding between aggressive cancer-directed treatment and best supportive care alone. Prognostic scoring systems exist for brain metastasis but not for LMD.
The Graded Prognostic Assessment (GPA) initially developed for brain metastases was later refined into the updated diagnosis-
specific GPA (DS-GPA) in 2012, a validated tool widely used to predict the median survival in brain metastases from lung cancer, breast cancer, melanoma, renal cell carcinoma, and gastrointestinal cancers. It utilizes a distinct combination of the validated prognostic factors: age, KPS, presence of extracranial metastasis, and number of brain metastases for each cancer type (Table 1).8 The DS-GPA tool helps clinicians estimate prognosis and make treatment decisions through a standardized patient-specific prognosis assessment in brain metastases.
TABLE 1. DS-GPA Scoring Criteria to Estimate Survival From Brain Metastases
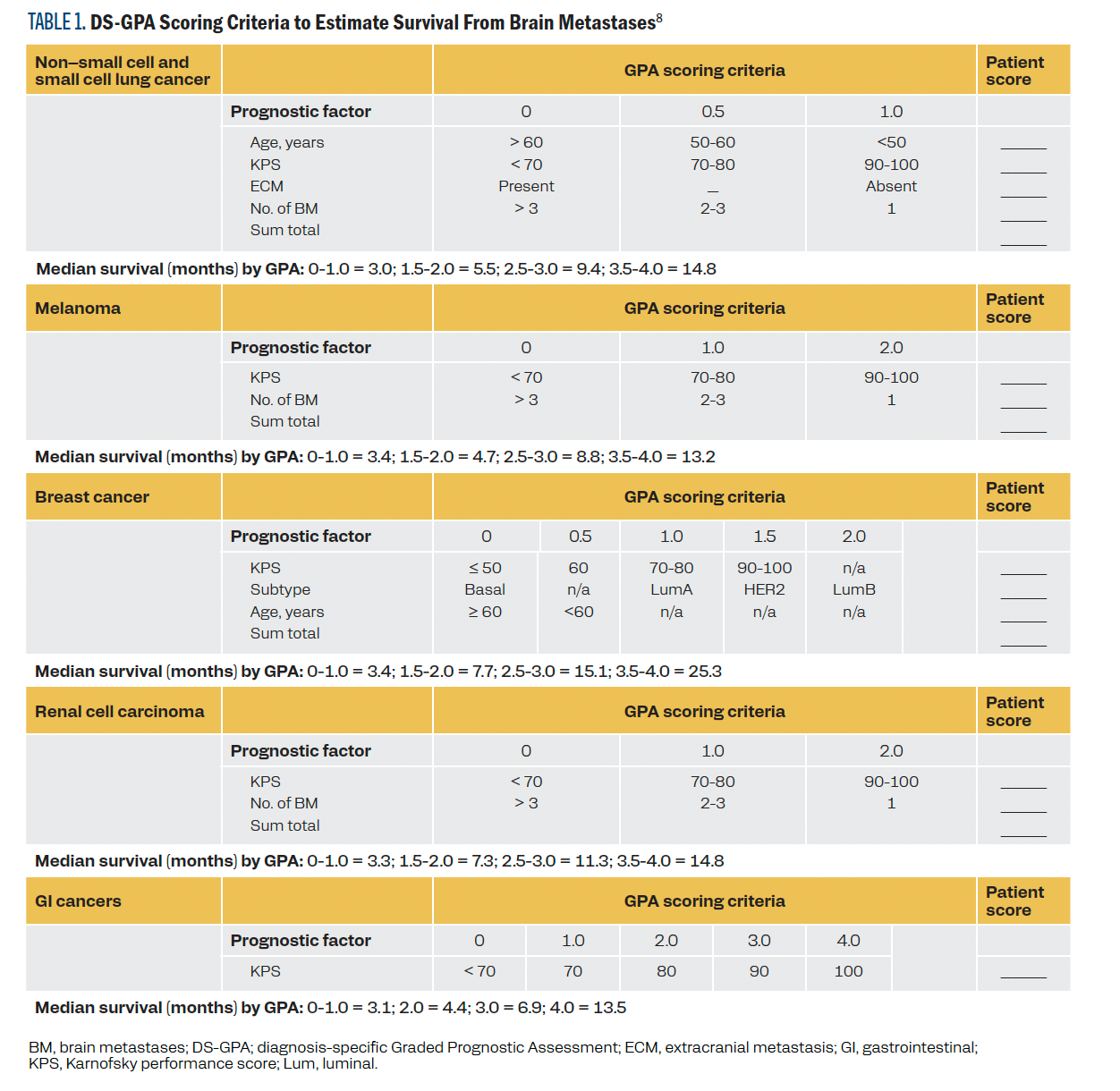
Our study assessed the utility of the DS-GPA tool and additional molecular and treatment variables to aid in the prognostication of LMD, given the lack of standard prognostic scoring systems for LMD.
Methods
We retrospectively reviewed the electronic health records of
82 adults diagnosed with LMD and treated at a single institution over a 5-year period. Sixty-four patients had cancer types represented in the DS-GPA; those with lung cancer (both small cell and non–small cell), melanoma, renal cell cancer, breast cancer, or gastrointestinal cancer were selected for analysis. The study received approval from the local institutional review board.
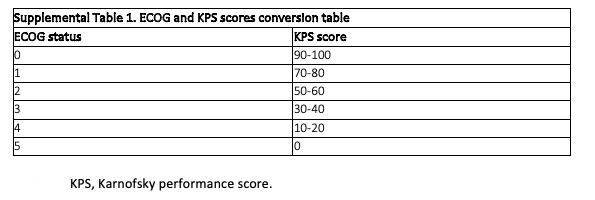
Patient and disease characteristics, LMD treatment, and survival data were collected for each patient. Also collected was each patient’s KPS score at the time of LMD diagnosis or within 2 months before LMD diagnosis. In cases where only ECOG status was available, the ECOG status was converted to a KPS score (supplemental Table 1). The type of LMD was defined as microscopic if CSF cytology was positive and as gross LMD if MRI imaging showed visible disease. Diffuse LMD was defined as LMD noted in 2 or more locations: the cerebrum, cerebellum, spine, or cranial nerves. DS-GPA scores were calculated using the cancer-specific calculator for each patient.8 Patients with breast cancer were classified based on molecular type as follows: luminal A subtype, highly estrogen receptor (ER) positive and progesterone receptor (PR) positive but negative for HER2; luminal B subtype, positive for ER, positive or negative for PR, and positive for HER2; basal subtype, negative for ER, PR, and HER2; and HER2-positive subtype, negative for ER and PR but positive for HER2.
Supplemental Table 1. ECOG and KPS Scores Conversion Table
Descriptive statistics, including counts, percentages, medians, and IQR, were used to summarize patient, cancer, and treatment characteristics. Overall survival (OS) was defined as the time from LMD diagnosis to death. Because all patients in this data set died within the follow-up period, no patients were censored. Differences in the OS among subgroups within a variable were assessed using log-rank tests. For categorical variables with more than 2 levels, P values were Bonferroni-adjusted to account for all possible pairwise comparisons. Cox proportional hazards model was used to assess whether the OS remained significantly different among subgroups of variables of interest after adjusting for cancer type (using stratification by cancer type). Proportional hazard assumptions were checked using Schoenfeld residuals. All analyses were conducted using SAS software version 9.4 (SAS Institute Inc).
Results
Patient, cancer, and treatment characteristics are summarized in Table 1. The median OS for all 64 patients, calculated from the time of LMD diagnosis, was 2.6 months (IQR, 1.1-6.2). There were no statistically significant differences in the OS among the different cancer types.
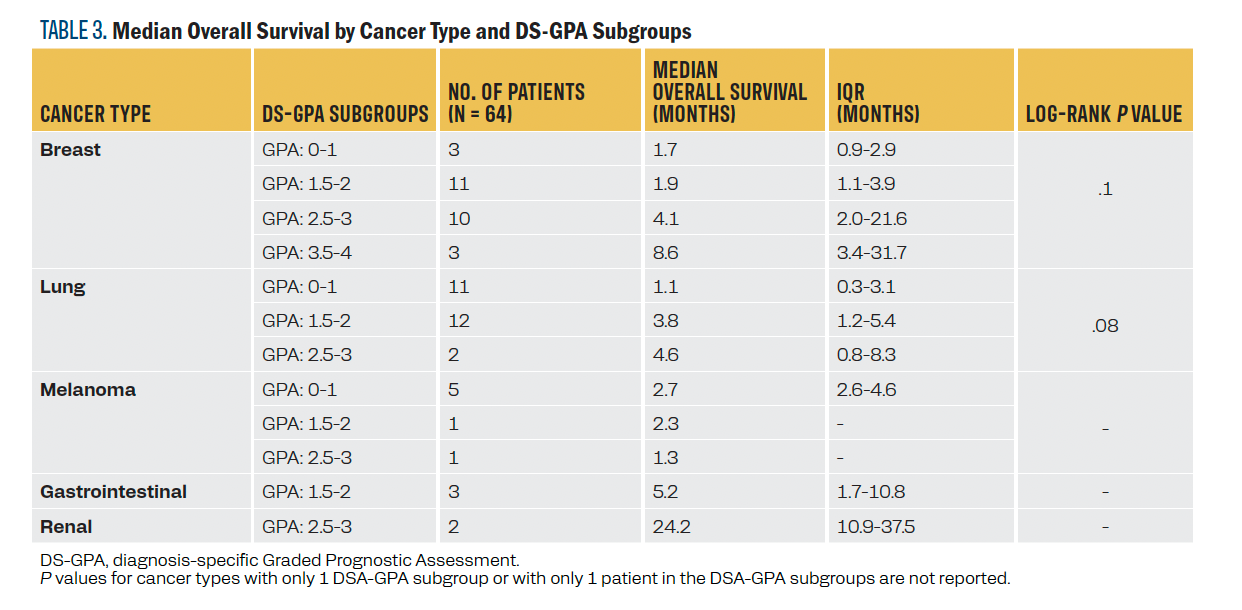
Although the differences did not reach statistical significance at the .05 α level, the DS-GPA appeared to differentiate survival at each score within the relatively larger breast and lung cancer cohorts (P = .10 and .08, respectively). In the breast cancer cohort, median survival improved with increasing GPA scores. Those with scores of 0 to 2 had a median OS of less than 2 months, whereas those with scores of 2.5 to 3 had a median OS of 4.1 months, and those with scores of 3.5 or higher had a median OS of 8.6 months. In the lung cancer cohort, 9 patients had small cell lung cancer (SCLC), and 16 had non–small lung cancer (NSCLC). Median OS improved with higher GPA scores: 1.1 months for a score of 0 to 1, 3.8 months for a score of 1.5 to 2, and 8.6 months for those with the highest score. Differences across DS-GPA subgroups for patients with melanoma, gastrointestinal cancer, and renal cell carcinoma could not be assessed due to their small sample sizes or lack of variability of DS-GPA within cancer type. For detailed median OS data for each cancer type and each DS-GPA subgroup within each cancer type, refer to Table 2 and Table 3, respectively.
TABLE 2. Characteristics and Median Overall Survival
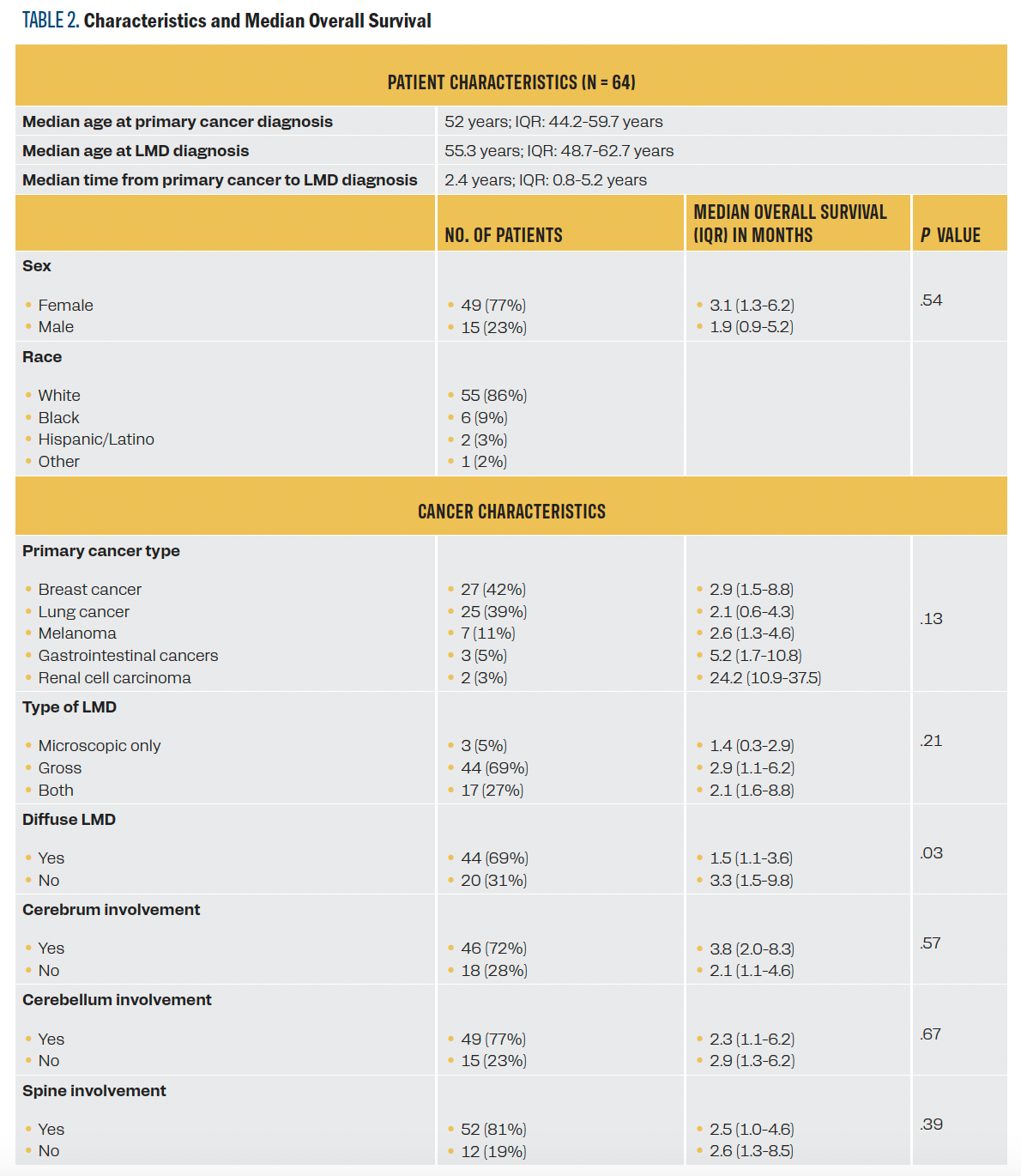
Table 2. Continued
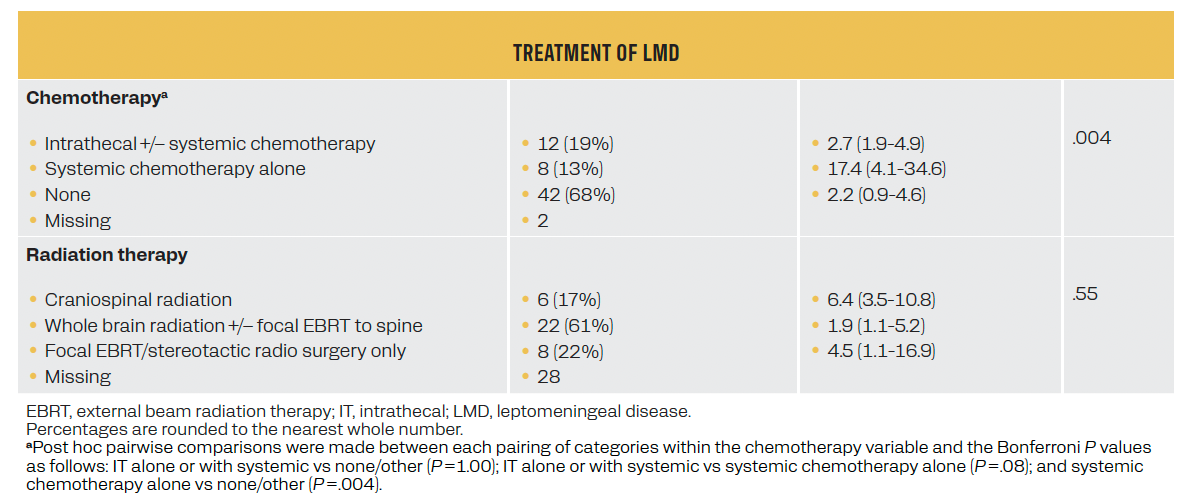
TABLE 3. Median Overall Survival by Cancer Type and DS-GPA Subgroups
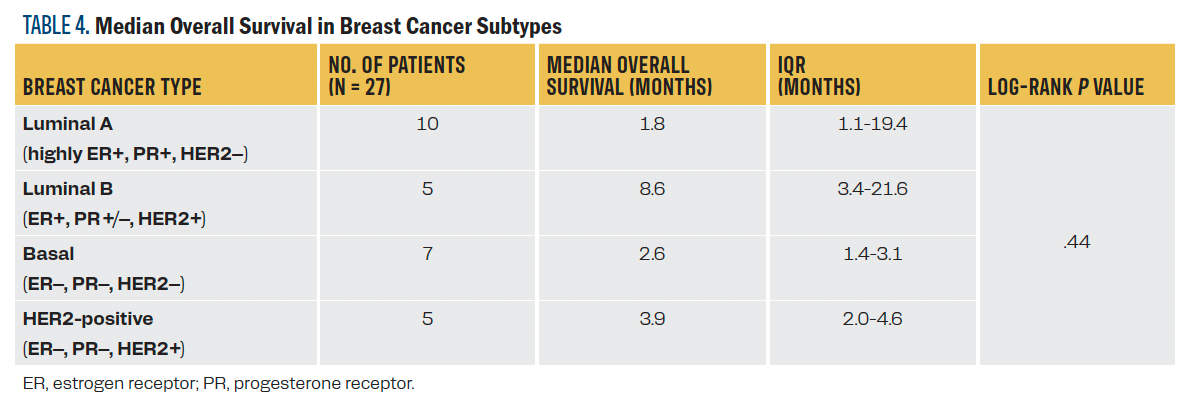
Among patients with breast cancer, luminal B (ER positive, PR positive/negative, and HER2 positive) patients had the longest median survival (8.6 months) followed by HER2 positive (3.9 months), although there were no significant differences in OS across the different breast cancer types (Table 4). Among the patients with NSCLC, 8 of 9 tested negative for EGFR mutation, and 8 of 8 patients tested negative for ALK translocations and ROS1 fusions.
TABLE 4. Median Overall Survival in Breast Cancer Subtypes
Patients without diffuse LMD and those receiving systemic chemotherapy alone vs intrathecal (IT) chemotherapy with or without systemic therapy had statistically longer survival (P = .03 and .004 respectively). Both associations remained significant after stratifying by cancer type (P = .048 and .03, respectively). Sex, type (gross, microscopic, both), location of LMD (cerebral, cerebellar, spine), and type of radiation were not associated with survival.
Discussion
The DS-GPA tool employs a scoring system that assigns points for each prognostic factor specific to a cancer type, categorizing individuals with brain metastases into DS-GPA subgroups validated with specific survival. A DS-GPA score of 4 correlates with the best prognosis, whereas a score of 0 indicates the worst prognosis.8 We found no statistically significant differences in OS among different DS-GPA subgroups for LMD occurring in the context of these malignancies. However, for cancer types with sufficient sample sizes, trends were observed where survival increased with increasing DS-GPA. This suggests that the DS-GPA tool may effectively prognosticate survival in LMD, with a much larger cohort needed to accurately differentiate survival groups within such a dismal survival range.
The median OS of all patients was 2.6 months from the time of LMD diagnosis, with no statistically significant differences in survival among primary cancer types. Historically, those with LMD from breast cancer have a better survival compared with those with other cancers. For instance, results from one retrospective study of 90 patients with LMD from 1975 to 1980 showed that 15% of patients with primary breast cancer were alive 1 year post treatment compared with 0% of those with lung cancer or melanoma.9 Similarly, results from another retrospective study of 155 patients with LMD from 1980 to 2002 showed the longest median OS of 11.3 months for breast cancer vs 4.7 months for melanoma,
1.8 months for lung cancer, and 2.8 months for other solid cancers.10 However, results from a retrospective study of 85 patients with LMD from 1997 to 2005 showed that although breast cancer was associated with a good prognosis in the univariate analysis, it lost its significance in the multivariate analysis, with only poor performance status and MRI-proven/gross LMD being associated with poor prognosis in the multivariate analysis.7 This implies that more effective systemic therapies and better performance status likely resulted in better survival in breast cancer rather than the primary cancer site or biology itself.11 Similarly, results from another retrospective study of 135 patients with LMD from 1989 to 2005 showed that although lung cancer and melanoma carried a poor prognosis in the univariate analysis, the significance of primary tumor type was abrogated by systemic chemotherapy.4 The absence of statistically significant differences in survival among the different primary tumors in our study may be due to modern treatment advancements, particularly therapies with better central nervous system (CNS) activity, even for non–breast cancers, as our study population is more contemporary (2012-2017). However, the small sample size in the gastrointestinal and renal cancer subtypes likely also affected the results, especially considering potential outliers with longer survival skewing the median survival for these cancers.
Breast cancer treatment and prognosis depend on the molecular subtypes. Per the DS-GPA, patients with brain metastases from luminal B breast cancer have a survival advantage, followed by those with HER2-positive cancer, and this pattern was similar in our LMD cohort as well. In patients with LMD and breast cancer, those with HR-positive/negative and HER2-positive disease have the longest median OS from LMD diagnosis (4.4-5.2 months, followed by those with HR-positive and HER2-negative disease (3.7-3.9 months). Those patients with HR-negative and HER2-negative disease have the worst survival (2.2-2.5 months).12,13 This disparity in survival among the subtypes is likely attributed to the availability and efficacy of several HER2-targeted systemic drugs, which possess better blood-brain penetration and, thus, are more effective in treating patients with LMD.14 In addition to the systemic HER2-targeted drugs, IT trastuzumab, whether given alone or as part of combination therapies, also has been shown to improve median survival to 13.5 months.15 In our study, the median OS of all patients with breast cancer was 2.9 months from LMD diagnosis. Although not statistically significant, patients with luminal B (ER-positive, PR-positive/negative, and HER2-positive disease) had a trend toward longer survival (8.6 months), followed by those with HER2-positive disease (3.9 months), which supports similar prognostic differences for breast cancer subtypes in LMD. Those cancers with targeted therapy options are more effectively treated.
There has been significant progress in the treatment of patients with metastatic NSCLC with novel therapies targeting EGFR, ALK, and ROS1.16 In lung cancer, patients with LMD have a median OS of approximately 3 months, but it is much longer in those with targetable molecular alterations.17 Patients with EGFR-
mutated lung cancer with LMD treated with CNS-penetrant EGFR inhibitors had a median OS of 11 to 18 months, depending on the study.18-20 Patients with ALK-positive lung cancer treated with the ALK inhibitor ceritinib had a median OS of 7.2 months.21 In our study, the median OS of all patients with lung cancer was
2.1 months. Among those tested for EGFR, ALK, and ROS1 alterations, only 1 had an EGFR mutation, but this patient survived less than 1 month. Because of the lack of patients with targetable alterations, we could not assess differences in OS in those with and those without targetable alterations. Further investigation is needed to understand the prognostic implications of targetable alterations in LMD from lung cancer.
Those without diffuse LMD had a significantly longer median survival (P = .03), which supports a correlation between lower LMD burden and improved outcomes, commensurate with prior studies correlating CSF protein, clinical neuropathies, and gross seeding on MRI with shorter survival. When assessing the route of chemotherapy, the post hoc pairwise comparisons showed that those treated with systemic chemotherapy alone had significantly longer survival than those treated with IT chemotherapy with or without systemic therapy. The most important finding was that survival was not better in those treated with IT chemotherapy. These findings align with previous studies’ results indicating no neurological response or survival benefit with IT chemotherapy, particularly in breast cancer, highlighting its controversial role.22-24
Across all solid cancers, systemic chemotherapy combined with involved field radiation therapy yielded comparable outcomes with lower toxicity compared with the addition of IT chemotherapy to systemic chemotherapy and involved field radiation.25 Similarly, results of another study of patients with hematologic and solid cancers showed that those who received systemic chemotherapy, either as a single treatment or as a combined treatment modality, had longer median OS compared with those receiving other treatment modalities or best supportive care alone.4 The superior outcomes with systemic chemotherapy are likely a result of improved CNS penetration and activity of more modern therapies, and effective control of the systemic disease. The lack of significant benefit with IT chemotherapy is likely due to limited drug options for IT administration, inadequate drug penetration into the deep layers of the leptomeningeal tumor masses, and uneven drug distribution due to frequent CSF flow obstruction.26
In our study, sex, type, location of LMD, and type of radiation therapy did not significantly impact OS. Although investigators in one study identified being female as an independent positive predictor of survival irrespective of the primary cancer type, a higher percentage of females opting for aggressive therapies compared with males in that study may have confounded the results, as other studies have failed to show sex as a prognostic factor.4-7 Prior studies have shown poorer survival for those with gross LMD, but ours did not, possibly due to a paucity of patients with only microscopic disease.7 Survival did not differ by the location of LMD along the craniospinal axis, although investigators in another study found clinical involvement of cerebral leptomeninges to be a negative predictor of survival, likely due to the higher LMD burden given the clinical symptoms rather than the location of LMD itself.5 The majority of our patients received whole brain radiation therapy with or without focused external beam radiation therapy (EBRT) to the spine, without any survival differences among those who received craniospinal radiation, focal EBRT, or stereotactic radiation. Although we are missing radiation therapy data for 28 patients, this aligns with results from prior studies showing similar survival improvement with any form of radiation over best supportive care alone.27
The main limitations of our study are: (1) a small sample size, particularly in melanoma, gastrointestinal cancers, and renal cell carcinoma to assess the utility of DS-GPA; (2) the majority of patients did not receive any route of chemotherapy (67.7%); (3) radiation therapy details were absent for 28 patients (43.8%) before the incorporation of that department into our electronic medical record system; (4) a potential selection bias, as treatment may have been provided to only those with good performance and expected better prognosis, which could have affected the survival outcomes; (5) a single-center retrospective study limits the generalizability of our study findings because of the potential unique patient demographics, diagnostic, and treatment practices in our institution compared with others.
Conclusion
The DS-GPA shows potential promise as a tool to delineate prognostic differences within breast and lung cancer cohorts with LMD. Larger, multi-institutional modern patient cohorts are necessary to determine whether the DS-GPA can accurately predict survival in LMD and whether incorporating disease burden and systemic chemotherapy will further strengthen its accuracy.
CORRESPONDING AUTHOR
Meghana Kesireddy, MBBS
University of Nebraska Medical Center
Omaha, NE 68198
Phone: 402-559-5600
Email: megahana.kesireddy@unmc.edu
References
- Groves MD. Leptomeningeal disease. Neurosurg Clin N Am. 2011;22(1):67-78, vii. doi:10.1016/j.nec.2010.08.006
- Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int. 2013;4(suppl 4):S265-S288. doi:10.4103/2152-7806.111304
- Kaplan JG, DeSouza TG, Farkash A, et al. Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol. 1990;9(3):225-229. doi:10.1007/BF02341153
- Oechsle K, Lange-Brock V, Kruell A, Bokemeyer C, de Wit M. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: a retrospective analysis. J Cancer Res Clin Oncol. 2010;136(11):1729-1735. doi:10.1007/s00432-010-0831-x
- Balm M, Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol. 1996;53(7):626-632. doi:10.1001/archneur.1996.00550070064013
- Palma JA, Fernandez-Torron R, Esteve-Belloch P, et al. Leptomeningeal carcinomatosis: prognostic value of clinical, cerebrospinal fluid, and neuroimaging features. Clin Neurol Neurosurg. 2013;115(1):19-25. doi:10.1016/j.clineuro.2012.03.048
- Waki F, Ando M, Takashima A, et al. Prognostic factors and clinical outcomes in patients with leptomeningeal metastasis from solid tumors. J Neurooncol. 2009;93(2):205-212. doi:10.1007/s11060-008-9758-3
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419-425. doi:10.1200/JCO.2011.38.0527
- Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49(4):759-772. doi:10.1002/1097-0142(19820215)49:4<759::aid-cncr2820490427>3.0.co;2-7
- Herrlinger U, Förschler H, Küker W, et al. Leptomeningeal metastasis: survival and prognostic factors in 155 patients. J Neurol Sci. 2004;223(2):167-178. doi:10.1016/j.jns.2004.05.008
- Rudnicka H, Niwińska A, Murawska M. Breast cancer leptomeningeal metastasis--the role of multimodality treatment. J Neurooncol. 2007;84(1):57-62. doi:10.1007/s11060-007-9340-4
- Morikawa A, Jordan L, Rozner R, et al. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer. 2017;17(1):23-28. doi:10.1016/j.clbc.2016.07.002
- Abouharb S, Ensor J, Loghin ME, et al. Leptomeningeal disease and breast cancer: the importance of tumor subtype. Breast Cancer Res Treat. 2014;146(3):477-486. doi:10.1007/s10549-014-3054-z
- Krishnan M, Krishnamurthy J, Shonka N. Targeting the sanctuary site: options when breast cancer metastasizes to the brain. Oncology (Williston Park). 2019;33(8):683730
- Zagouri F, Sergentanis TN, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13-22. doi:10.1007/s10549-013-2525-y
- Tan AC, Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol. 2022;40(6):611-625. doi:10.1200/JCO.21.01626
- Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7(2):382-385. doi:10.1097/JTO.0b013e3182398e4f
- Flippot R, Biondani P, Auclin E, et al. Activity of EGFR tyrosine kinase inhibitors in NSCLC with refractory leptomeningeal metastases. J Thorac Oncol. 2019;14(8):1400-1407. doi:10.1016/j.jtho.2019.05.007
- Yang JCH, Kim SW, Kim DW, et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol. 2020;38(6):538-547. doi:10.1200/JCO.19.00457
- Ahn MJ, Chiu CH, Cheng Y, et al. Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: the AURA leptomeningeal metastases analysis. J Thorac Oncol. 2020;15(4):637-648. doi:10.1016/j.jtho.2019.12.113
- Chow LQM, Barlesi F, Bertino EM, et al. ASCEND-7: efficacy and safety of ceritinib treatment in patients with ALK-positive non-small cell lung cancer metastatic to the brain and/or leptomeninges. Clin Cancer Res. 2022;28(12):2506-2516. doi:10.1158/1078-0432.CCR-21-1838
- Boogerd W, Hart AA, van der Sande JJ, Engelsman E. Meningeal carcinomatosis in breast cancer. Prognostic factors and influence of treatment. Cancer. 1991;67(6):1685-1695. doi:10.1002/1097-0142(19910315)67:6<1685::aid-cncr2820670635>3.0.co;2-m
- Boogerd W, van den Bent MJ, Koehler PJ, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer. 2004;40(18):2726-2733. doi:10.1016/j.ejca.2004.08.012
- Orlando L, Curigliano G, Colleoni M, et al. Intrathecal chemotherapy in carcinomatous meningitis from breast cancer. Anticancer Res. 2002;22(5):3057-3059.
- Bokstein F, Lossos A, Siegal T. Leptomeningeal metastases from solid tumors: a comparison of two prospective series treated with and without intra-cerebrospinal fluid chemotherapy. Cancer. 1998;82(9):1756-1763.
- Ushio Y, Shimizu K, Aragaki Y, Arita N, Hayakawa T, Mogami H. Alteration of blood-CSF barrier by tumor invasion into the meninges. J Neurosurg. 1981;55(3):445-449. doi:10.3171/jns.1981.55.3.0445
- Buszek SM, Chung C. Radiotherapy in leptomeningeal disease: a systematic review of randomized and non-randomized trials. Front Oncol. 2019;9:1224. doi:10.3389/fonc.2019.01224

How Supportive Care Methods Can Improve Oncology Outcomes
Experts discussed supportive care and why it should be integrated into standard oncology care.