3 Things You Should Know About TROP2 as a Therapeutic Target in Triple-Negative Breast Cancer
Read an expert-led article on the latest advancements in TROP2-targeted antibody-drug conjugates for treating triple-negative breast cancer and improving patient outcomes.
The authors

LEARNING OBJECTIVES
Upon successful completion of this activity, you should be better prepared to:
• Evaluate efficacy and safety data from clinical trials evaluating TROP2-targeted antibody-drug conjugates (ADCs) for triple-negative breast cancer (TNBC)
• Integrate TROP2-targeted ADCs into individualized treatment plans for patients with TNBC
RELEASE DATE: September 1, 2025
EXPIRATION DATE: September 1, 2026
Accreditation/Credit Designation
Physicians’ Education Resource®, LLC, is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. Physicians’ Education Resource®, LLC, designates this enduring material for a maximum of 0.25 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Acknowledgement of Commercial Support
This activity is supported by an educational grant from Gilead Sciences, Inc.
Off-Label Disclosure and Disclaimer
This activity may or may not discuss investigational, unapproved, or off-label use of drugs. Learners are advised to consult prescribing information for any products discussed. The information provided in this activity is for accredited continuing education purposes only and is not meant to substitute for the independent clinical judgment of a health care professional relative to diagnostic, treatment, or management options for a specific patient’s medical condition. The opinions expressed in the content are solely those of the individual faculty members, and do not reflect those of PER® or any company that provided commercial support for this activity.
INSTRUCTIONS FOR PARTICIPATION and HOW TO RECEIVE CREDIT
1. Read this activity in its entirety.
2. Go to https://www.gotoper.com/ibc25trop2tnbc-postref to access and complete the posttest.
3. Answer the evaluation questions.
4. Request credit using the drop-down menu.
YOU MAY IMMEDIATELY DOWNLOAD YOUR CERTIFICATE.
Antibody-drug conjugates (ADCs) deliver potent chemotherapy directly to cancer cells by harnessing the targeting ability of monoclonal antibodies. Linking the cytotoxic drug to the antibody enhances its half-life and broadens the therapeutic window, allowing more cancer cells to reach the vulnerable mitotic phase for effective treatment. Here are 3 things you should know about the use of TROP2-targeting ADCs in the treatment of patients with triple-negative breast cancer (TNBC).
1) TROP2-targeting ADCs deliver potent cytotoxic drugs specifically to tumor cells.
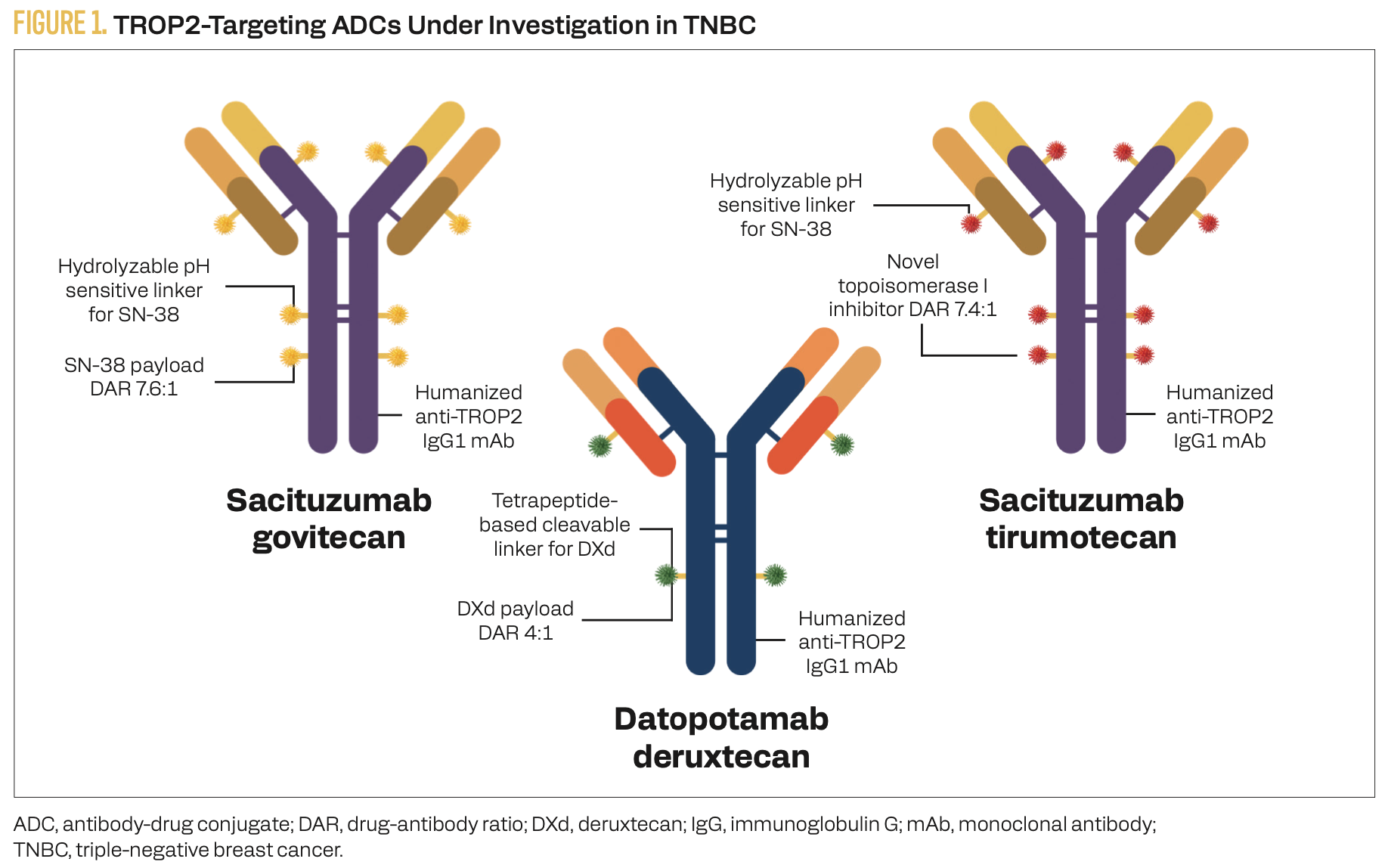
TROP2 is a cell surface antigen overexpressed in approximately 93% of TNBC tumors and shows low expression in most normal tissues, making it an attractive target for anticancer therapies.1 Sacituzumab govitecan (SG), datopotamab deruxtecan (Dato-DXd), and sacituzumab tirumotecan (sac-TMT) are TROP2-directed ADCs approved or under investigation for TNBC (Figure 1). Although each delivers a distinct cytotoxic payload, all 3 utilize a topoisomerase I inhibitor, which promotes single-strand DNA breaks and triggers cellular senescence or apoptosis. These ADCs differ in their average drug to antibody ratios (DARs): SG at 7.6:1, sac-TMT at 7.4:1, and Dato-DXd at 4:1. A higher DAR can enhance antitumor potency but may also increase the risk of off-target toxicity.
FIGURE 1. TROP2-Targeting ADCs Under Investigation in TNBC
2) Clinical trial designs are evaluating TROP2-targeting ADCs across a variety of patients and in combination with other therapies.
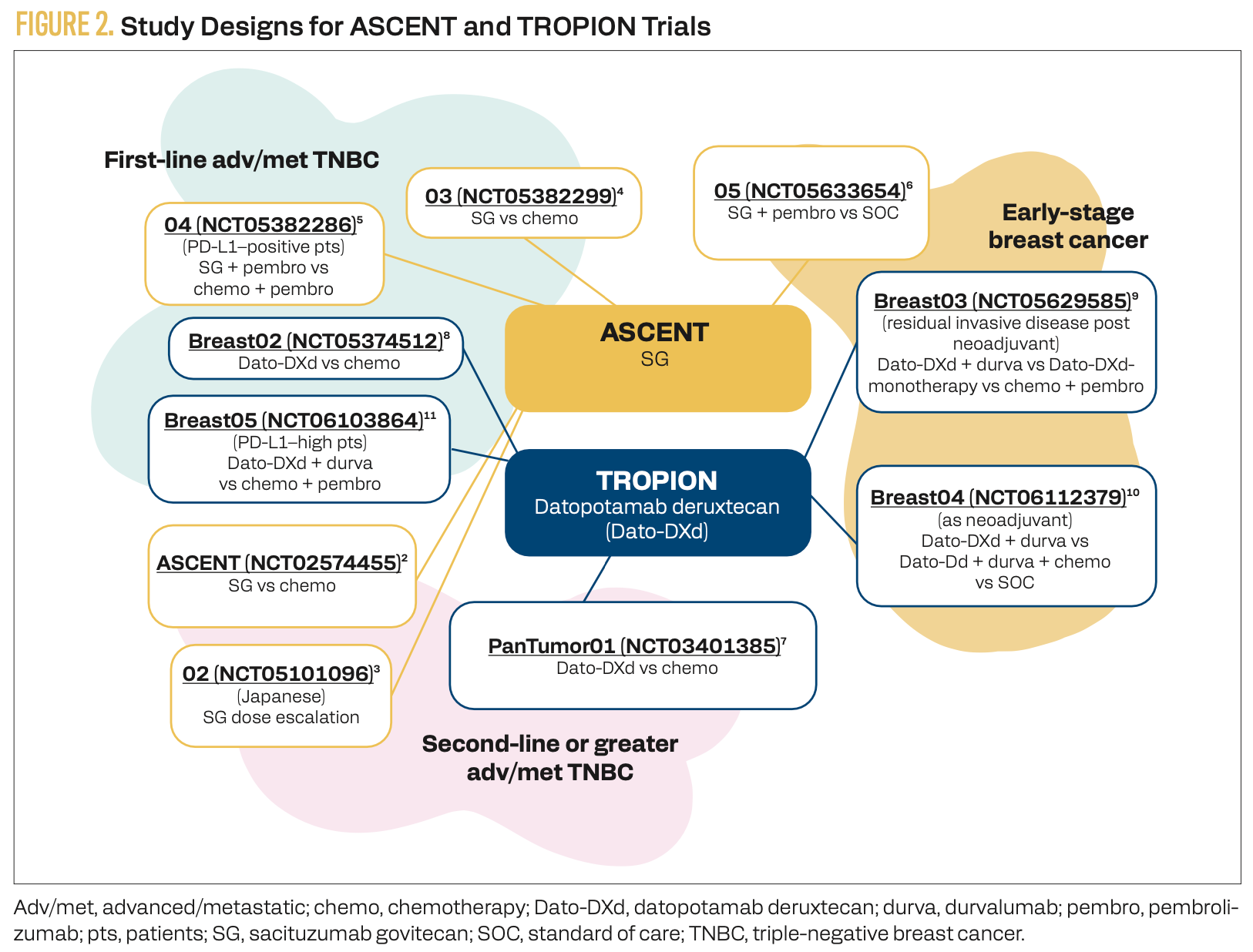
The efficacy of SG alone and combined with immune checkpoint inhibitors (ICIs) against breast cancers of all stages is under evaluation in the ASCENT clinical trials (Figure 2).2-6 Similarly, the TROPION clinical trials are evaluating the benefit of Dato-DXd alone and in combination with chemotherapy and/or ICIs in all stages of breast cancer (Figure 2).7-11
FIGURE 2. Study Designs for ASCENT and TROPION Trials
3) TROP2-targeting ADCs are improving outcomes in patients with TNBC.
TROP2-targeting ADCs used as monotherapy have demonstrated efficacy in previously treated metastatic TNBC (mTNBC).12 In the phase 3 ASCENT trial (NCT02574455), SG significantly improved both progression-free survival (PFS; HR, 0.41; 95% CI, 0.32-0.52) and overall survival (OS; HR, 0.51; 95% CI, 0.42-0.63) compared with physician’s choice of chemotherapy in relapsed or refractory mTNBC (Figure 2).2 Based on these results, SG has received regular FDA approval for the treatment of patients with TNBC.13 The phase 3 ASCENT-03 trial (NCT05382299) assessed SG monotherapy in patients who had not received prior systemic therapy (Figure 2).4 In this study, SG was compared with standard first-line chemotherapy in patients with PD-L1–positive mTNBC. A recent press release reported that the trial met its primary PFS end point, with results to be presented at an upcoming meeting.14
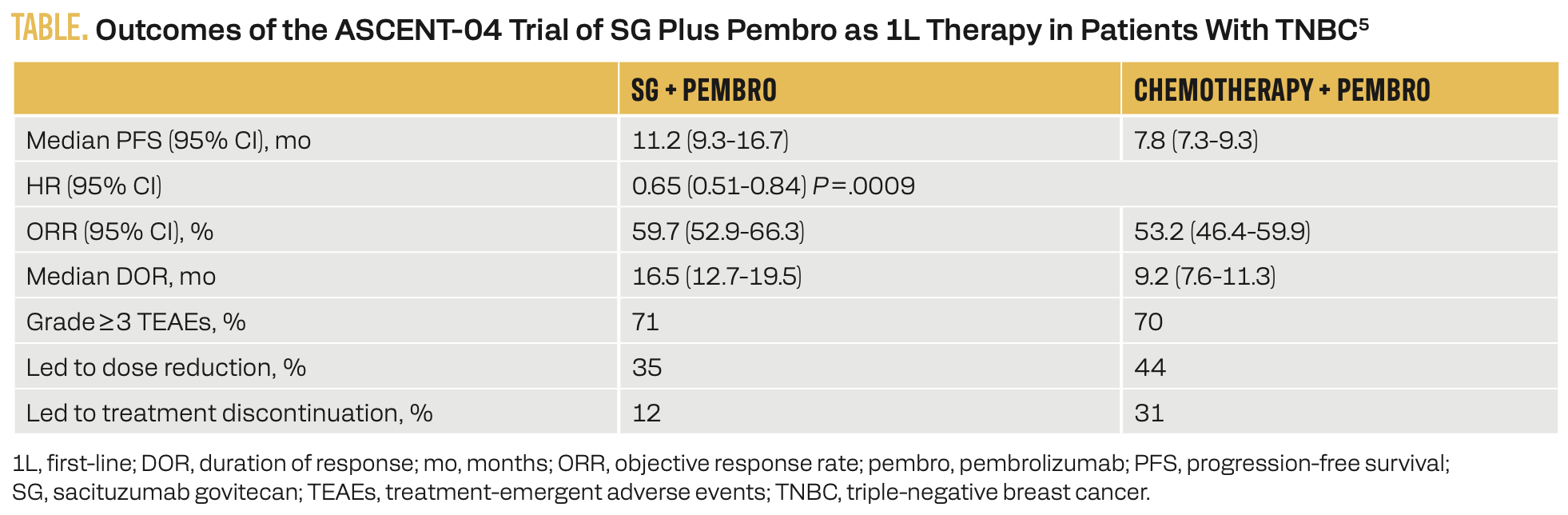
The phase 3 ASCENT-04 trial (NCT05382286) expanded upon ASCENT-03 by comparing first-line treatment with SG plus an ICI vs chemotherapy plus an ICI in patients with mTNBC (Figure 2).5 In this study, 443 patients were randomly assigned to receive either SG and pembrolizumab or physician’s choice of chemotherapy and pembrolizumab. The primary results are summarized in the Table.5
TABLE. Outcomes of the ASCENT-04 Trial of SG Plus Pembro as 1L Therapy in Patients With TNBC5
The combination of SG and pembrolizumab was also evaluated as neoadjuvant therapy in early-stage TNBC in the phase 2 NeoSTAR trial (NCT04230109).15 Among 48 patients with stage II disease and 2 with stage III disease, the pathologic complete response (pCR) rate was 34% (95% CI, 19.5%-45.7%). The overall response rate was 66% (95% CI, 50%-78%), including complete clinical response in 30% of patients. Additional neoadjuvant chemotherapy was administered to 26 patients, of whom 9 achieved pCR, bringing the total number of patients with pCR at surgery to 25 of 50. The most common adverse events (AEs) were nausea (56%), alopecia (52%), fatigue (46%), and diarrhea (44%). Four patients required dose reductions.
Dato-DXd monotherapy has also demonstrated efficacy in 44 previously treated patients with TNBC in the phase 1 TROPION-PanTumor01 trial (NCT03401385; Figure 2).7 The objective response rate (ORR) in this cohort was 31.8% (95% CI, 18.6%-47.6%), with a median duration of response of 16.8 months. Median PFS was 4.4 months (95% CI, 3.0-7.3). Stomatitis was the most common treatment-emergent AE, occurring in 72.7% of patients with TNBC, including grade 3 events in 11.4%. Dato-DXd monotherapy is being evaluated in comparison with chemotherapy as frontline therapy for patients with TNBC in the ongoing phase 3 TROPION-Breast02 trial (NCT05374512; Figure 2).8 Although Dato-DXd is approved for use in treating patients with hormone receptor–positive, HER2-negative breast cancer, it is still awaiting FDA approval for patients with TNBC.16
Sac-TMT is another TROP2-targeting ADC with a high DAR and a topoisomerase I inhibitor payload (Figure 1) that has demonstrated a benefit in TNBC. In the phase 3 OptiTROP-Breast01 trial (NCT05347134), sac-TMT significantly improved PFS (median, 6.7 vs 2.5 months; HR, 0.32; 95% CI, 0.24-0.44; P < .00001) and OS (median, not reached vs 9.4 months; HR, 0.53; 95% CI, 0.36-0.78; P = .0005) compared with chemotherapy in previously treated patients with TNBC.17 The ORR with sac-TMT was 45.4% vs 12.0% with chemotherapy. Grade 3 or higher treatment-related AEs (TRAEs) occurred in 63.1% of patients receiving sac-TMT and 56.8% receiving chemotherapy, leading to dose reductions and discontinuations in 30.8% and 1.5% of patients treated with sac-TMT and in 10.6% and 0.8% of patients in the chemotherapy arm, respectively.
The phase 2 OptiTROP-Breast05 trial (NCT05445908) evaluated sac-TMT as a first-line therapy in 41 patients with advanced or metastatic TNBC who had not received prior treatment.18 The ORR was 70.7% and the disease control rate was 92.7%. The median duration of response was 12.2 months, and the median PFS was 13.4 months. The most common grade 3 or higher TRAEs were decreased neutrophil count (46.3%), decreased lymphocyte count (7.3%), and fatigue (7.3%). Sac-TMT is approved for use in China, but it has not yet received FDA approval.19
Key References
5. Tolaney SM, Azambuja Ed, Kalinsky K, et al. Sacituzumab govitecan (SG) + pembrolizumab (pembro) vs chemotherapy (chemo) + pembro in previously untreated PD-L1–positive advanced triple-negative breast cancer (TNBC): primary results from the randomized phase 3 ASCENT-04/KEYNOTE-D19 study. J Clin Oncol. 2025;43(suppl 17):LBA109. doi:10.1200/JCO.2025.43.17_suppl.LBA109
7. Bardia A, Krop IE, Kogawa T, et al. Datopotamab deruxtecan in advanced or metastatic HR+/HER2- and triple-negative breast cancer: results from the phase I TROPION-PanTumor01 study.
J Clin Oncol. 2024;42(19):2281-2294. doi:10.1200/jco.23.01909
18. Yin Y, Ouyang Q, Yan M, et al. Sacituzumab tirumotecan (sac-TMT) as first-line treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): initial results from the phase II OptiTROP-Breast05 study. J Clin Oncol. 2025;43(suppl 16):1019. doi:10.1200/JCO.2025.43.16_suppl.1019
For full references list, visit https://www.gotoper.com/ibc25trop2tnbc-postref
CME Posttest Questions
1) Based on the primary analysis of the phase 3 ASCENT-04/KEYNOTE-D19 trial, which efficacy outcome(s) showed statistically significant improvement in patients with PD-L1–positive metastatic TNBC treated with sacituzumab govitecan (SG) plus pembrolizumab compared with chemotherapy plus pembrolizumab?
A. PFS only
B. OS only
C. Both PFS and OS
D. Neither PFS nor OS
2) A 43-year-old premenopausal woman with metastatic, PD-L1–negative, HER2 IHC 1+ TNBC received first-line gemcitabine/carboplatin with a duration of response of 18 months. She now presents with abdominal distention; imaging confirms disease progression with new liver lesions. AST/ALT and bilirubin levels are normal. Which of the following ADCs would you prioritize based on current phase 3 trial data?
A. Datopotamab deruxtecan
B. Sacituzumab govitecan
C. Trastuzumab deruxtecan
3) Based on the OptiTROP-Breast01 trial data, which efficacy outcome(s) showed statistically significant improvement with sacituzumab tirumotecan vs chemotherapy in pretreated metastatic TNBC?
A. PFS only
B. OS only
C. Both PFS and OS
D. Neither PFS nor OS
Claim Your CME Credit at https://www.gotoper.com/ibc25trop2tnbc-postref
To learn more about this topic, including key trial data for antibody-drug conjugates in patients with triple-negative breast cancer, go to https://www.gotoper.com/ibc25trop2tnbc-activity
CME Provider Contact information
Physicians’ Education Resource®, LLC
2 Commerce Drive, Suite 110, Cranbury, NJ 08512
Toll-Free: 888-949-0045
Local: 609-378-3701
Fax: 609-257-0705
info@gotoper.com
