Molecular Characterization of a Rare Glioblastoma Case With Atypical Histopathologic Features
A patient case of a 76-year-old man with a rare glioblastoma variant showcased the importance of molecular profiling in diagnosing and guiding treatment for brain tumors.
A patient case of a 76-year-old man with a rare glioblastoma variant showcased the importance of molecular profiling in diagnosing and guiding treatment for brain tumors.
Case description
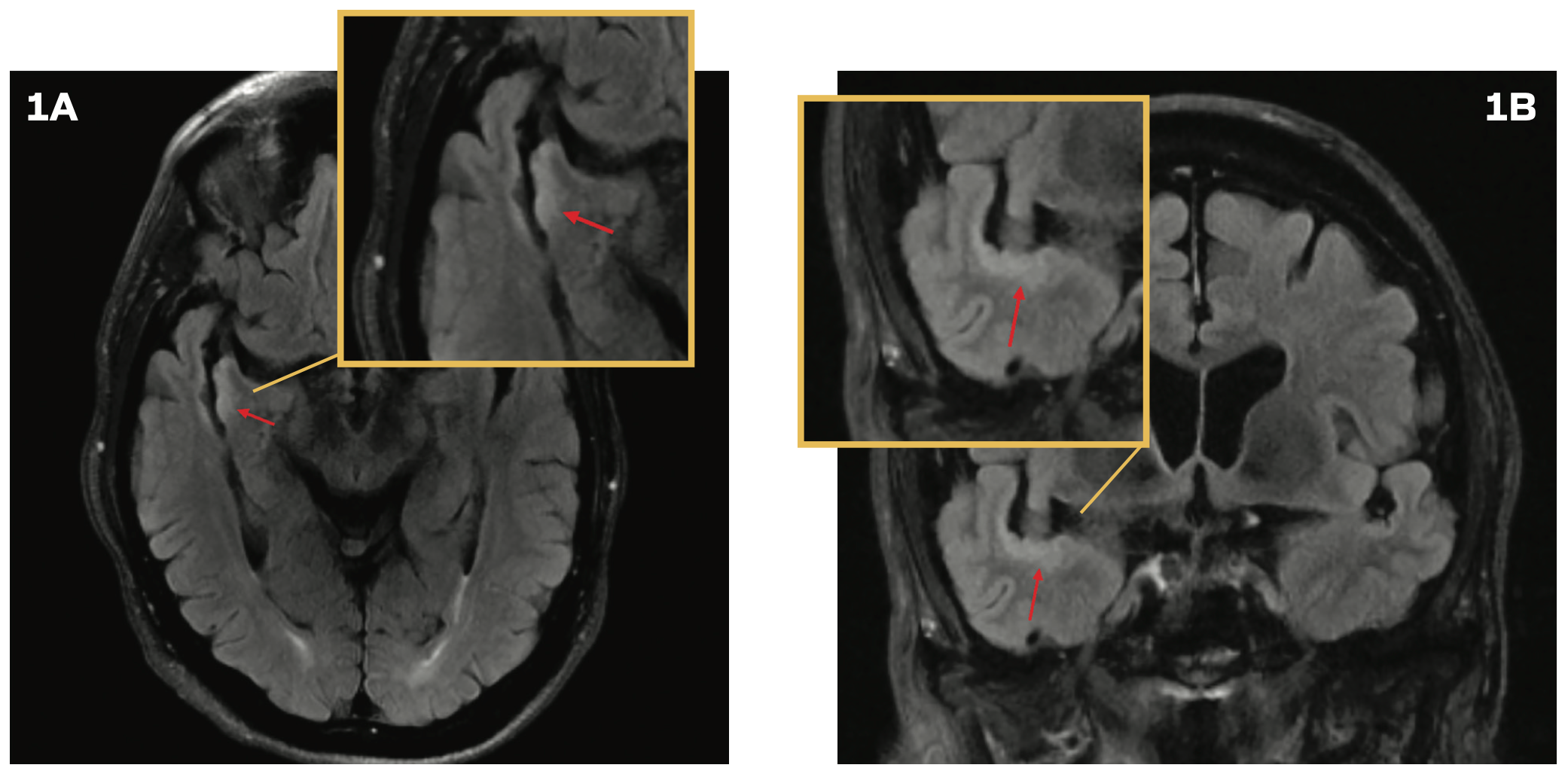
A 76-year-old man was evaluated in the emergency department following a mechanical fall that resulted in a head injury. He experienced a loss of consciousness for approximately 30 seconds. A CT scan of the head revealed no abnormalities. However, the patient continued to experience persistent headaches and dizziness. A brain MRI demonstrated subtle cortical thickening and a contiguous, non–mass-like increased T2 FLAIR signal along the temporal opercular and right insular cortex, without associated enhancement or restricted diffusion (Figures 1A and 1B), prompting a referral to neurosurgery for further evaluation. Subsequent brain MRIs performed 6, 12, and 22 months following the initial imaging did not show progression. However, at a 2.5-year
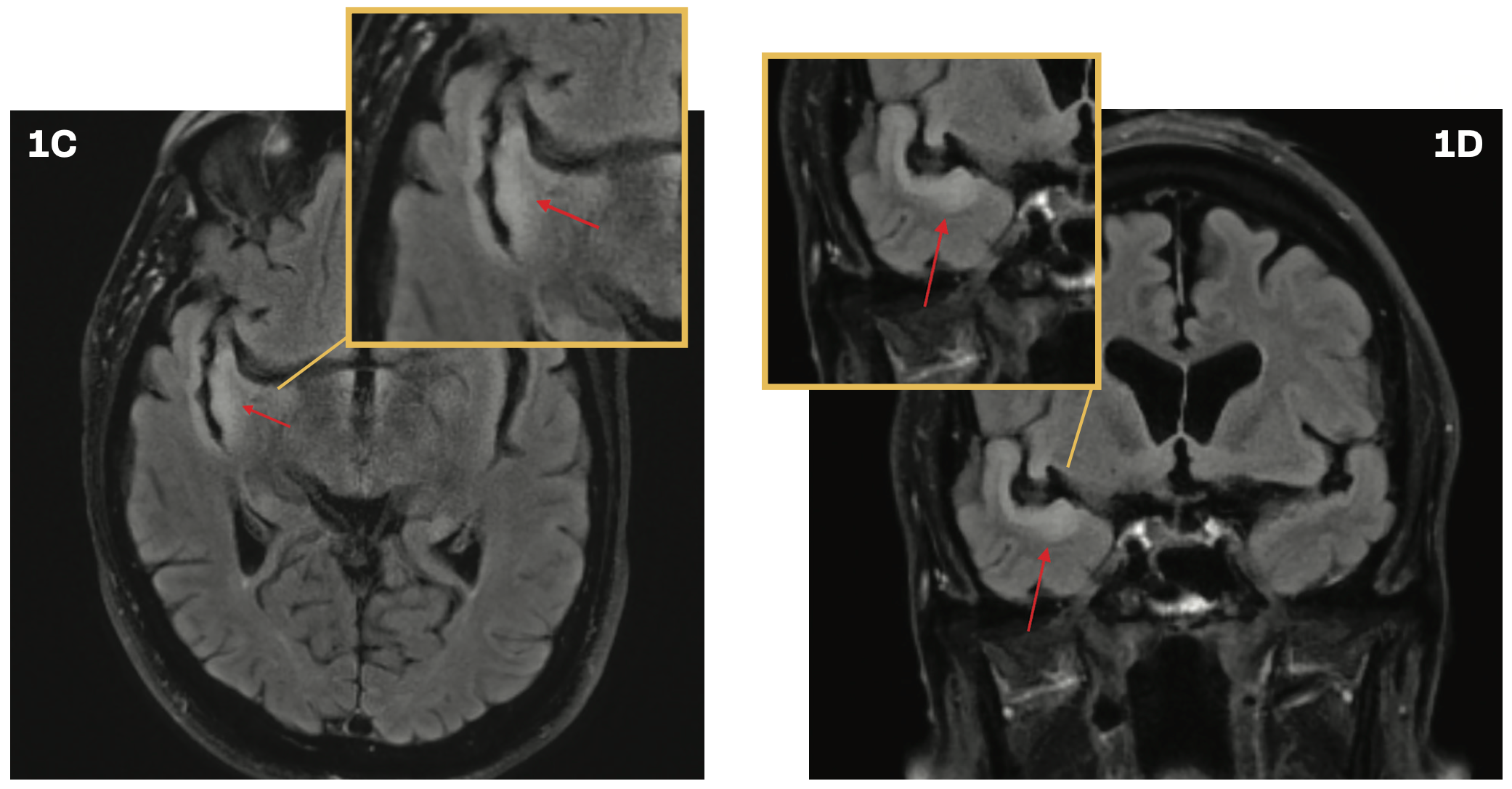
follow-up, brain imaging revealed progression of the T2 hyperintense abnormality along the ribbon of the superior temporal gyrus (Figures 1C and 1D). The radiographic findings suggested a potential low-grade glioma.
FIGURES 1A AND 1B. Brain MRI With Cortical Thickening
FIGURES 1C AND 1D. Brain Imaging at 2.5 Years’ Follow-Up
Testing Options
A transsylvian approach for resection was recommended based on the radiographic progression, and the patient underwent the surgery. Intraoperative frozen section diagnosis was consistent with an infiltrating glioma, without high-grade features. The most posterior component of the insular lesion was obscured by overlying vascular structures, and no safe dissection corridor could be identified. Given the intraoperative impression of near-total resection, the decision was made to halt the resection. Postoperative imaging showed hemorrhage in the sylvian fissure, with subsequent involution on follow-up MRI, and no enhancing mass. The patient’s postoperative course was complicated by a surgical wound infection and dehiscence, which was managed with debridement and antibiotics, resulting in the resolution of the infection.
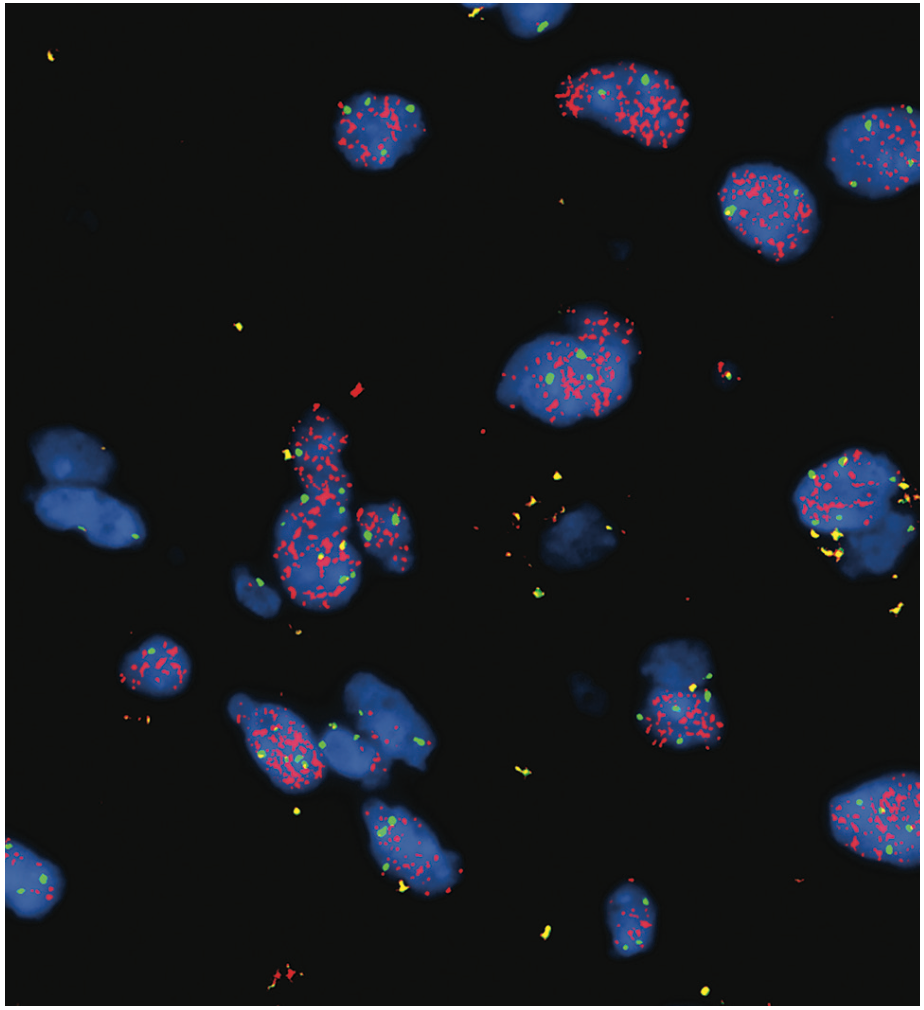
Hematoxylin and eosin staining of the surgical specimen demonstrated mildly hypercellular atypical glial cells without evidence of increased mitotic figures or necrosis (Figure 2A). Ki-67 labeling index was low, approximately 1% (Figure 2B). Immunohistochemistry for IDH1 R132H was negative. Next-generation sequencing (NGS) was performed for further characterization, revealing the following key findings: TERT c.-146C> T mutation with 26% allele frequency, EGFR amplification with an estimated copy number of 158 (confirmed by fluorescence in situ hybridization with an EGFR signal/control signal ratio of 7.6; see Figure 3), and CDKN2A deletion. National Institutes of Health methylation profiling was performed, which indicated a consensus match to glioblastoma, IDH wild-type, with a high confidence score. This was supported by the presence of the +7/–10 chromosomal copy number change, TERT promoter mutation, and the high-level EGFR amplification, confirming that the neoplasm exhibited the molecular features of glioblastoma. O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation analysis showed negative methylation status.
FIGURE 2A. Hematoxylin and Eosin Staining
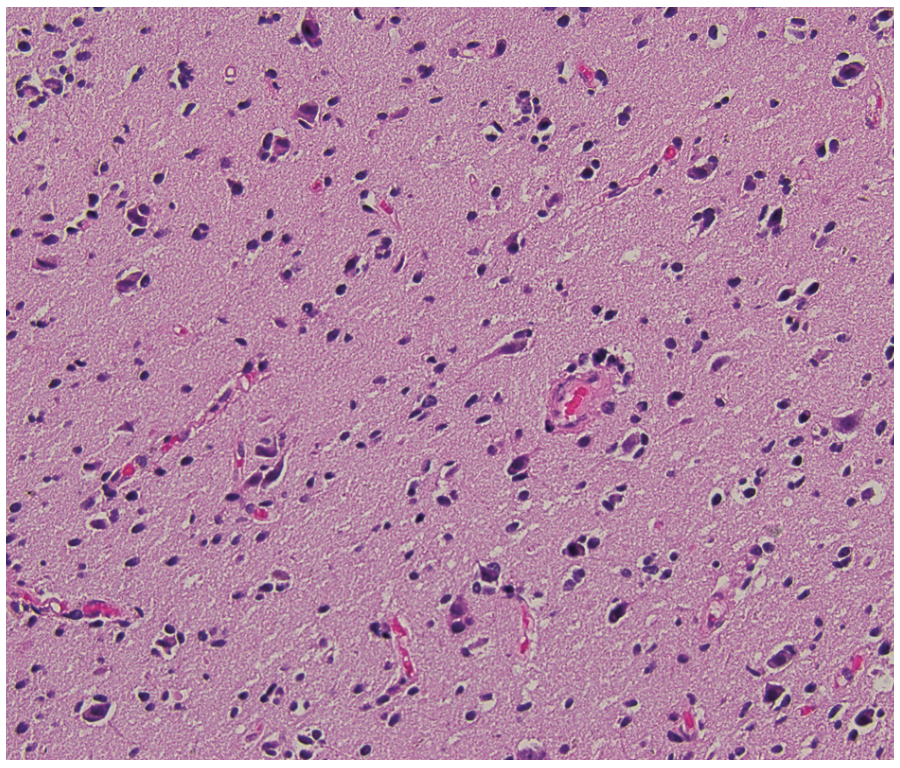
FIGURE 2B. Ki-67 Index
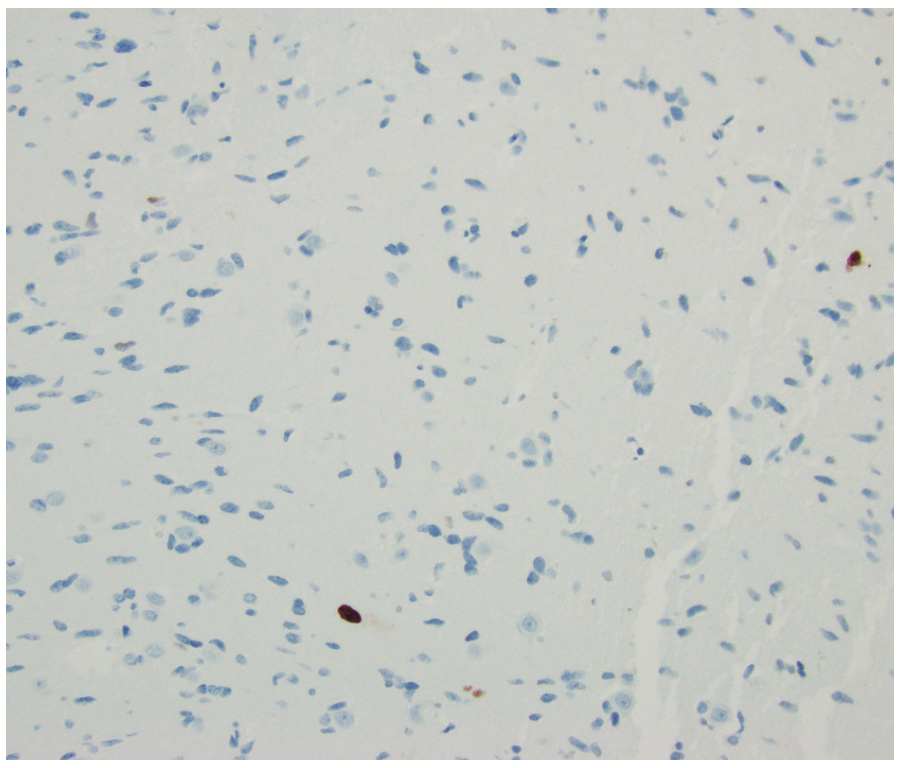
FIGURE 3. Fluorescence in Situ Hybridization With EGFR Signal/Control
Diagnosis
These findings were consistent with a diagnosis of molecular glioblastoma, IDH wild-type, central nervous system (CNS) World Health Organization (WHO) grade 4. Although the histology showed mildly hypercellular and atypical neuroglial tissue with low Ki-67 proliferative index and inconspicuous mitotic activity, the molecular findings of TERT promoter mutation and EGFR amplification, and the methylation profiling support the above diagnosis. The patient was referred to the medical oncology and radiation oncology departments for recommendations regarding adjuvant treatment. He completed adjuvant therapy consisting of 5250 cGy hypofractionated radiation with concurrent temozolomide (75 mg/m² daily), followed by maintenance temozolomide and tumor treating fields.
Discussion
Diffuse gliomas are a group of primary CNS tumors characterized by the widespread infiltration of glial cells in the brain. The classification of diffuse gliomas has evolved significantly in recent years, incorporating molecular biomarkers alongside traditional histological features to better predict clinical outcomes and guide treatment decisions. The 2021 WHO update places great emphasis on molecular testing for diagnosis and grading.1 Additionally, DNA methylation profiling has emerged as an important tool for identifying specific tumor types based on their methylation patterns.2The presence of EGFR amplification, TERT promoter mutation, or +7/–10 copy number alterations classifies IDH wild-type gliomas as glioblastoma, IDH wild-type, WHO grade 4, according to the 2021 WHO and College of American Pathologists guidelines, regardless of histological features.3
Glioblastoma is a highly aggressive primary brain tumor with a dismal prognosis and an average survival of 15 to 18 months. The standard treatment approach involves maximal surgical resection, followed by chemoradiotherapy and maintenance chemotherapy, with tumor treating fields sometimes used as an adjunct.4 The lack of progression over such an extended period, as seen in this case, is highly atypical for glioblastoma.
Ki-67 proliferation index correlates with the grade of diffuse gliomas, with higher-grade gliomas typically exhibiting elevated Ki-67 levels.5 However, its prognostic significance remains uncertain. In IDH wild-type glioblastomas, the median Ki-67 labeling index is approximately 25%. The exceptionally low Ki-67 index observed in this case is rare for glioblastoma. Furthermore, none of the classic histological features of glioblastoma, such as marked hypercellularity, microvascular proliferation, or necrosis, were present in this case.
The integration of NGS and methylation profiling proved to be essential tools in diagnosing this challenging case and led to the classification of the tumor as glioblastoma, grade 4, despite the presence of otherwise atypical histological features. This molecular insight was pivotal in guiding treatment, underscoring the critical role of advanced genetic profiling.
This case may represent an early, incidental detection of a glioblastoma multiforme precursor lesion, with molecular drivers that could have led to the development of the more typical histological form in the future. Alternatively, it may be a distinct variant of glioblastoma with slow clinical progression and atypical histological features. NGS did not reveal a TP53 mutation or MDM2 amplification. The lack of a TP53 mutation indicates this is likely a de novo glioblastoma. Further research is needed to explore the molecular patterns in atypical glial cells. A better understanding of their behavior and evolution will enhance our knowledge of the diagnostic and prognostic significance of these findings and help guide evidence-based treatment strategies.
Corresponding Author
Nanuli Gvazava, MD
Kansas University Medical Center
References
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231-1251. doi:10.1093/neuonc/noab106
- Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469-474. doi:10.1038/nature26000
- Brat DJ, Aldape K, Bridge JA, et al. Molecular biomarker testing for the diagnosis of diffuse gliomas. Arch Pathol Lab Med. 2022;146(5):547-574. doi:10.5858/arpa.2021-0295-CP
- Kotecha R, Odia Y, Khosla AA, Ahluwalia MS. Key clinical principles in the management of glioblastoma. JCO Oncol Pract. 2023;19(4):180-189. doi:10.1200/OP.22.00476
- Dahlrot RH, Bangsø JA, Petersen JK, et al. Prognostic role of Ki-67 in glioblastomas excluding contribution from non-neoplastic cells. Sci Rep. 2021;11(1):17918. doi:10.1038/s41598-021-95958-9
