3 Things You Should Know About Data Presented at ESMO GI
New therapies for hepatobiliary cancers show promise, expanding treatment options for patients with advanced disease and challenging conditions.
The panel.

Patients with hepatobiliary cancers (HBCs) have low 5-year survival rates across all stages of disease, underscoring the unmet need for improved local and systemic therapies.1,2 Patients with certain features often seen in HBCs, including Child-Pugh (CP) class B moderate liver dysfunction, main trunk portal vein thrombosis (Vp4), poor ECOG performance status (PS), and bulky disease, have been underrepresented in clinical trials.3-5 Exciting new data presented at the European Society of Medical Oncology (ESMO) Gastrointestinal Cancers Congress 2025 demonstrated that access to effective HBC therapies could potentially be expanded to patients who do not meet the usual inclusion criteria for pivotal trials. Here are 3 things you should know about the recently presented data.
1. Frontline dual checkpoint inhibition is effective treatment for patients with unresectable hepatocellular carcinoma (uHCC) and feasible for some with moderate liver dysfunction, portal vein thrombosis, or poor ECOG PS.
Following the demonstration of efficacy of second-line ipilimumab plus nivolumab (ipi/nivo) in the phase 1/2 CheckMate040 study (NCT01658878), investigators in the phase 3 CheckMate-9DW study (NCT04039607) evaluated the regimen vs lenvatinib or sorafenib as frontline treatment for patients with uHCC.6,7 Patients receiving ipi/nivo had a higher objective response rate (ORR; 36% vs 13%; P < .0001) and reduced risk of health-related quality-of-life deterioration (HR for Functional Assessment of Cancer Therapy–Hepatobiliary total score, 0.69; 95% CI, 0.55-0.87) vs treatment with lenvatinib or sorafenib.7,8 Approximately 30% of study participants in the ipi/nivo arm required high-dose steroids for immune-related adverse events (AEs), most of which occurred during the first 3 months of treatment.8 Encouragingly, receipt of steroids during the study period did not appear to substantially worsen overall survival (OS; 27.9 vs 23.7 months for the ipi/nivo arm overall vs for those who received high-dose steroids).8,9
The phase 3b SIERRA study (NCT05883644) evaluated co-primary end points of possibly treatment-related grade 3 or 4 AEs (PRAEs) and the ORR for 98 patients with uHCC receiving the STRIDE regimen (a single dose of tremelimumab plus regular-interval ongoing durvalumab) in the frontline setting.10 However, unlike the CheckMate-9DW and phase 3 HIMALAYA (NCT03298451) studies, SIERRA included patients with CP B7 or B8 liver disease with an ECOG PS of 0 or 1 (n = 35), CP class A liver disease with an ECOG PS of 2 (n = 44), or with HCC with chronic Vp4 (n = 19).9-12 Grade 3 or 4 PRAEs occurring within 6 months of treatment initiation, AEs leading to discontinuation of any treatment, and PRAEs leading to death were reported in 19.4%, 10.2%, and 2.0% of patients, respectively. These data suggest that cancer-directed treatment for patients with HCC and moderate liver dysfunction (Figure 1), ECOG PS of 2, or main portal vein thrombus is safe and feasible.3
FIGURE 1. Balancing Liver Function and HCC Treatment for Patients With Moderate Liver Dysfunction3
2. Durvalumab can be combined with several different gemcitabine-based regimens, including noncisplatin regimens, and infused over a shortened 30-minute interval to safely and effectively treat advanced biliary tract cancer (aBTC).
The phase 3 TOPAZ-1 study (NCT03875235) data demonstrated that adding durvalumab to first-line gemcitabine plus cisplatin improves ORR (26.7% vs 18.7%) and OS (median, 12.9 vs 11.3 months; HR, 0.74; 95% CI, 0.63-0.87) compared with chemotherapy alone.13,14 The phase 3b TOURMALINE study (NCT05771480) also evaluated the efficacy of frontline durvalumab plus chemotherapy for patients with aBTC.15,16 However, unlike TOPAZ-1, the TOURMALINE study included noncisplatin chemotherapy regimens and enrolled patients who had an ECOG PS of 2, ampulla of Vater cancer, or disease recurring less than 6 months after surgery or adjuvant treatment (Figure 2).1,15-17 Safety was manageable for all 7 arms of the TOURMALINE study, which included durvalumab plus gemcitabine alone or in combination with S-1, oxaliplatin, carboplatin, cisplatin, cisplatin plus paclitaxel, or cisplatin plus S-1.15,16 Higher ORRs were observed in platinum vs nonplatinum regimens (25.8% vs 4.2%) and platinum doublet vs platinum triplet regimens (29.3% vs 20.5%).16 The initial 60-minute infusion of durvalumab also was compared with subsequent 30-minute infusions on the study, and no difference in infusion safety was observed between the 30-minute and 60-minute infusions.15 Rather, the risk of infusion-related AEs was limited to those patients who received some form of platinum-based chemotherapy, whereas no infusion-related AEs were observed in patients receiving nonplatinum regimens.
FIGURE 2. Anatomic Locations of Biliary Tract Cancers Included in the TOURMALINE Study16,17

3. Patients with advanced HCC, including those with bulky disease, benefit from combining systemic therapy with transarterial chemoembolization (TACE).
The treatment paradigm for HCC has included local therapies for Barcelona Clinic Liver Cancer (BCLC) stage A disease (early) and systemic therapy for BCLC stage C disease (advanced).18 Data from several studies have recently demonstrated that adding immune checkpoint inhibitorcombinations to TACE for patients with BCLC stage B disease (intermediate) confers a progression-free survival (PFS) benefit.19-21
The phase 3 EMERALD-1 study (NCT03778957) compared TACE alone vs in combination with durvalumab plus bevacizumab in patients with uHCC with an ECOG PS of 0 or 1 and CP A-B7 liver function.19 The primary end point of PFS was significantly improved with the addition of systemic therapy (median PFS, 15.0 vs 8.2 months; HR, 0.77; 95% CI, 0.61-0.98; P = .032; significance threshold, P = .0434).
The phase 3 LEAP-012 study (NCT04246177) compared TACE plus pembrolizumab and lenvatinib vs TACE alone in patients with incurable, intermediate-stage HCC, CP A liver function, and an ECOG PS of 0 or 1.20 The PFS was significantly improved for the group who received systemic therapy in addition to TACE (median PFS, 14.6 vs 10.0 months; HR, 0.66; 95% CI, 0.51-0.84; P = .0002; significance threshold, P = .025).However, OS data were immature and did not meet the threshold for significance.
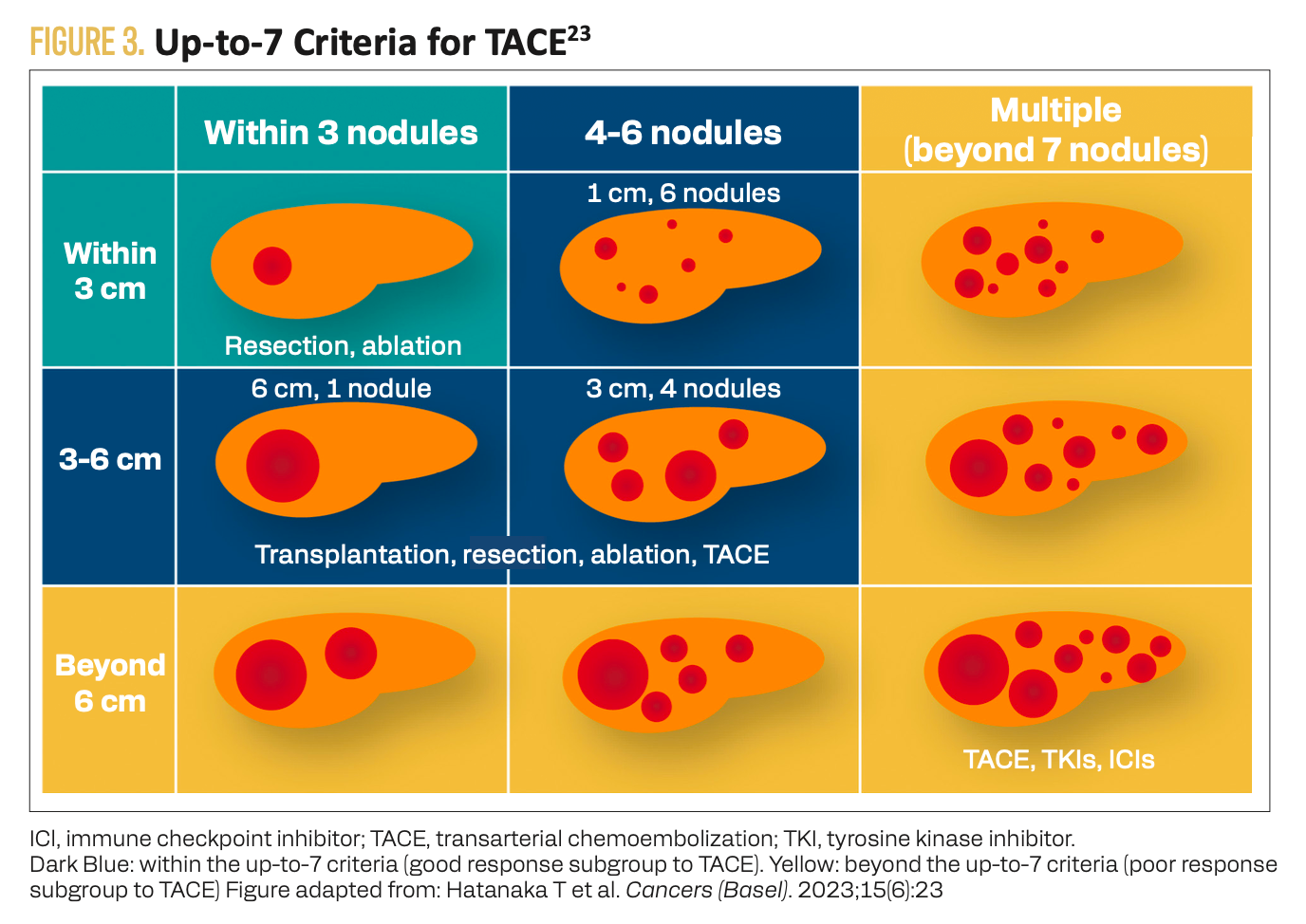
The phase 3 TALENTACE study (NCT04712643) evaluated on-demand TACE alone vs with atezolizumab plus bevacizumab in uHCC.21 Approximately half the patients evaluated in the EMERALD-1 and LEAP-012 studies had bulky disease (tumor burden beyond the up-to-7 criteria or tumor burden score higher than 6), whereas a sum of the maximum tumor diameter plus number of lesions totaling 6 or more was one of the key inclusion criteria for TALENTACE (Figure 3).19,20,22,23 The primary study end point of TACE-PFS (time from randomization to unTACEable progression, TACE failure/refractoriness, or any-cause death) was significantly improved for patients who received atezolizumab plus bevacizumab in addition to on-demand TACE (median, 11.30 vs 7.03 months; HR, 0.71; 95% CI, 0.55-0.92; 2-sided P = .009).21 The rate of grade 3 to 5 TRAEs was 63.8% vs 42.2% in the TACE plus atezolizumab and bevacizumab vs TACE alone groups, respectively.
FIGURE 3. Up-to-7 Criteria for TACE23
Key References
10. Chan SL, Kudo M, Sangro B, et al. Safety results from the phase IIIb SIERRA study of durvalumab (D) and tremelimumab (T) as first-line (1L) treatment (tx) for hepatocellular carcinoma (HCC) participants (pts) with a poor prognosis.Ann Oncol. 2025;36(suppl 1):S63-S64. doi:10.1016/j.annonc.2025.05.163
16. Oh DY, Ikeda M, Mercade TM, et al. Early safety and efficacy from the phase IIIb TOURMALINE study of durvalumab (D) in combination with gemcitabine (G)-based chemotherapy in advanced biliary tract cancer (aBTC). Ann Oncol. 2025;36(suppl 1):S126. doi:10.1016/j.annonc.2025.05.338
21. Dong J, Han G, Ogasawara S, et al. TALENTACE: a phase III, open-label, randomized study of on-demand transarterial chemoembolization (TACE) combined with atezolizumab + bevacizumab (Atezo+Bev) or on-demand TACE alone in patients with systemically untreated, intermediate-to-high burden unresectable hepatocellular carcinoma (uHCC). Ann Oncol. 2025;36(suppl 1):S62. doi:10.1016/j.annonc.2025.05.542
For full references list, visit https://www.gotoper.com/oth25esmogi-postref
CME Posttest Questions
1. Which of the following first-line immunotherapy-based regimens has been shown to be feasible and tolerable in patients with advanced HCC and main trunk portal vein thrombosis (Vp4) but good performance status and contraindications to bevacizumab?
A. Nivolumab + ipilimumab
B. Pembrolizumab + lenvatinib
C. Tremelimumab for 1 dose plus ongoing durvalumab (STRIDE)
D. Immunotherapy-based treatment is contraindicated in Vp4 disease
2. The randomized, phase 3 TALENTACE study enrolled patients with unresectable HCC and high tumor burden. Compared with observation, what was the impact of adding atezolizumab-bevacizumab to on-demand TACE in terms of the composite TACE-PFS end point?
A. Worse median TACE-PFS
B. No significant difference in median TACE-PFS
C. Improved median TACE-PFS
3. In the phase 3b TOURMALINE study, which evaluated durvalumab plus investigator’s choice chemotherapy for patients with advanced biliary tract cancers, which of the following factors was associated with the incidence of infusion-related reactions?
A. Female sex
B. Nonmetastatic disease
C. Shortening durvalumab infusion time from 60 minutes to 30 minutes
D. Treating with a platinum-based chemotherapy regimen
Claim Your CME Credit at https://www.gotoper.com/oth25esmogi-postref
To learn more about this topic, including information on FGFR inhibition in advanced HCC, PARP inhibition for advanced BTC with homologous recombination repair deficiency, and immunotherapy for advanced BTC, go to https://www.gotoper.com/oth25esmogi-activity
CME Provider Contact Information
Physicians’ Education Resource®, LLC
259 Prospsect Plains Rd,
Building H
Monroe, NJ 08831
Toll-Free: 888-949-0045
Local: 609-378-3701
Fax: 609-257-0705
info@gotoper.com
