Bisphosphonates in the Prevention and Treatment of Bone Metastases
Bisphosphonates have an established role in treating tumor-inducedhypercalcemia and decreasing the incidence of skeletal-related events.Recent data suggest that these agents may also prevent skeletal metastases.This review explains how cancer metastasizes to bone and howbisphosphonates may block this process, with a summary of clinicaltrials supporting the use of bisphosphonates to treat and prevent bonemetastases. For skeletal metastases in patients with breast cancer,multiple myeloma, or other solid tumors, bisphosphonates are importantadjuncts to systemic therapy. Despite promising results in metastaticprostate cancer, additional trials are needed before bisphosphonatesbecome part of standard treatment in this setting. Ongoing trials areevaluating the preventive role of the third-generation bisphosphonatesin breast cancer patients. Until the results of these trials are presented,bisphosphonates should only become a component of adjuvant treatmentin the context of a clinical trial. Bone loss, a common consequenceof cancer treatment, should be treated with the usual measures indicatedfor the management of osteoporosis, including bisphosphonates.
ABSTRACT: Bisphosphonates have an established role in treating tumor-induced hypercalcemia and decreasing the incidence of skeletal-related events. Recent data suggest that these agents may also prevent skeletal metastases. This review explains how cancer metastasizes to bone and how bisphosphonates may block this process, with a summary of clinical trials supporting the use of bisphosphonates to treat and prevent bone metastases. For skeletal metastases in patients with breast cancer, multiple myeloma, or other solid tumors, bisphosphonates are important adjuncts to systemic therapy. Despite promising results in metastatic prostate cancer, additional trials are needed before bisphosphonates become part of standard treatment in this setting. Ongoing trials are evaluating the preventive role of the third-generation bisphosphonates in breast cancer patients. Until the results of these trials are presented, bisphosphonates should only become a component of adjuvant treatment in the context of a clinical trial. Bone loss, a common consequence of cancer treatment, should be treated with the usual measures indicated for the management of osteoporosis, including bisphosphonates.
The skeleton is a frequent site of metastases in human cancer, and as such is associated with morbid skeletal-related events such as pain, pathologic fractures, hypercalcemia, and cord compression. Bisphosphonates, specific inhibitors of osteoclasts, have an established role in the treatment of tumor-induced hypercalcemia and in decreasing the frequency of skeletal-related events. More recently, evidence is emerging that these agents may prevent skeletal metastases. This review summarizes what happens when cancer metastasizes to bone, how bisphosphonates work, and the clinical trials that support the use of bisphosphonates in the treatment and prevention of bone metastases.
Pathophysiology of Skeletal Metastases
Normal bone is maintained by a dynamic balance between the cells that breakdown or resorb bone (osteoclasts) and the cells that form new bone (osteoblasts).[1] Bone breakdown and new bone formation is constantly ongoing in discrete areas called remodeling units. The regulation of the remodeling unit occurs at several levels. First, the force of gravity puts mechanical stress on bone. Without gravity, as in space, humans lose bone-a major problem for astronauts in prolonged spaceflight. Second, circulating hormones (including parathyroid hormones, calcitonin, insulin, thyroid hormones, vitamin D, sex steroid hormones, and growth hormones) stimulate bone breakdown or new bone formation. Third, the autocrine and paracrine factors are derived from osteoclasts, osteoblasts, and stromal cells in the bone microenvironmment. Gravity, circulating hormones, and most importantly, factors within the remodeling unit orchestrate the activity of osteoclasts and osteoblasts to maintain the balance between breakdown and new bone formation.
The autocrine and paracrine factors that maintain the balance between resorption and new bone formation include transforming growth factors (TGFs), insulin-like growth factors (IGFs), platelet-derived growth factors (PDGFs), tumor necrosis factors (TNFs), interleukins (ILs),[1] and the more recently identified receptor activator of nuclear factor-kappa B ligand (RANK-L), RANK receptor, and osteoprotegerin.[2,3] These factors not only maintain the normal balance, but some (ie, IGFs, PDGFs, TGFs, TNFs) are growth factors for tumors. The bone microenvironment is rich in the relevant factors that support tumor growth, and this may explain why, for example, breast cancer, prostate cancer, and multiple myeloma have a high frequency of skeletal metastases.
FIGURE 1
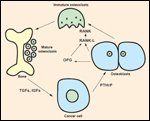
Schematic Representation of Osteoclast-Induced Bone Resorption
RANK-L and osteoprotegerin play central roles in regulating bone resorption. RANK-L drives resorption, osteoprotegerin blocks resorption, and the ratio between them governs normal remodeling, benign metabolic bone disease, and tumor-related osteoclast activation.[3] When tumors metastasize to bone, they produce parathyroid hormone-related protein that stimulates the osteoblasts to produce RANK-L.[4,5] The RANK receptor on the osteoclast precursors is activated by ligand binding and causes immature precursor cells to grow and differentiate into mature osteoclasts (Figure 1). With increased resorption, more tumor growth factors are released, causing a "vicious cycle" of osteoclast activation.[5,6] The increased resorption leads to the clinical sequelae of pain, hypercalcemia, and pathologic fracture, as well as the appearance of a lytic lesion on the skeletal radiograph. Osteoprotegerin is a soluble receptor produced by osteoblasts and stromal cells in the bone marrow. Osteoprotegerin binds to RANK-L and inactivates it, limiting RANK receptor activation and, in turn, resportion.
Increased osteoblastic activity is found in skeletal metastases from prostate cancer. This manifests as new bone formation or sclerosis on the skeletal radiograph. Prostate cancer cells produce the fibroblast growth factor (FGF), bone morphogenetic protein, PDGF, endothelin-1, TGFbeta, and IGF that increase osteoblast activity.[9] Likewise, they produce proteases such as urokinase-type plasminogen activator and prostatespecific antigen, which activate latent TGF-beta and IGF from the bone microenvironment. These support the further growth of prostate cancer cells-hence, another vicious cycle.[10,11]
Action of Bisphosphonates
FIGURE 2
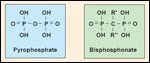
Structure of Inorganic Pyrophosphate and Bisphosphonate
Bisphosphonates are synthetic analogs of inorganic pyrophosphate (Figure 2). Substituting the central oxygen atom for carbon renders the bisphosphonate structure more stable and resistant to thermal, chemical, and enzymatic degradation. The side-chain substitutions (R1 and R2) of the carbon atom with halogen, sulfur, nitrogen, hydroxyl, or other groups, result in a wide range of structures with varying antiresorptive potencies (Table 1).
Bisphosphonates are specific inhibitors of osteoclasts and act via several mechanisms. These include inhibition of cancer cells binding to the bone matrix[12,13]; inactivation of the adenosine triphosphate-dependent proton pump, which inhibits the secretion of protons required to dissolve the bone mineral matrix[14]; disruption of the osteoclast cytoskeleton[15]; induction of apoptosis of osteoclasts[ 16,17]; inhibition of the recruitment and differentiation of osteoclast precursors[18]; inhibition of matrix metalloproteinases[19,20]; and direct antiproliferative and proapoptotic effects on tumor cells, both in vitro and in vivo.[21-25]
TABLE 1

Relative Resorptive Potency of Bisphosphonates
Pamidronate, a second-generation bisphosphonate, and zoledronate (zoledronic acid, Zometa), a third-generation bisphosphonate, have been approved by the Food and Drug Administration for the treatment of hypercalcemia of malignancy and lytic skeletal metastases from breast cancer and multiple myeloma; zoledronate is also indicated for patients with other solid tumors. Clodronate is not approved in the United States but is available in Europe.
Bisphosphonates are not metabolized. When administered intravenously (IV), clearance from the plasma is rapid, but the majority of the drug is taken up by the skeleton, where it remains for a prolonged period. About 20% to 40% of these drugs is excreted unchanged in the urine 24 hours after administration.[1] When administered as an oral formulation, bisphosphonates are poorly absorbed from the gastrointestinal tract with limited bioavailability.
Pamidronate and zoledronate are relatively well tolerated with few side effects.[26,27] Fever, myalgia, arthralgia, headache, diarrhea, asymptomatic hypocalcemia, and nausea are some of the common side effects following intravenous infusion. Severe toxic reactions, acute renal failure, aseptic peritonitis, and a variety of eye lesions have also been reported rarely.
Treatment of Hypercalcemia of Malignancy
Hypercalcemia of malignancy affects approximately 10% to 20% of all cancer patients; if untreated, the condition is potentially fatal.[28] The mechanism of hypercalcemia of malignancy involves osteoclast-mediated resorption via the action of parathyroid hormone-related protein. In some cancers, such as squamous cell carcinoma, hypercalcemia occurs in the absence of detectable skeletal metastases due to the release of parathyroid hormone-related protein into the circulation. The clinical symptoms of this disorder are nonspecific and include dehydration, weight loss, fatigue, loss of appetite, constipation, nausea, vomiting, and mental status changes. If unrecognized, it can cause seizures, renal failure, cardiac conduction abnormalities and arrhythmia, coma, and death.
TABLE 2
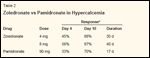
Zoledronate vs Pamidronate in Hypercalcemia
Along with rehydration, effective inhibition of the osteoclast-mediated bone resorption will result in rapid control of hypercalcemia of malignancy; thus, bisphosphonates are standard treatment for this disorder. Pamidronate is active,[29,30] but zoledronate is more effective.[31] Two randomized, concurrent, parallel phase III trials compared the safety and efficacy of IV zoledronate at 4 mg or 8 mg vs IV pamidronate at 90 mg for the treatment of hypercalcemia of malignancy.[31] Zoledronate was more effective in returning serum calcium levels to normal and maintaining them as such for a longer period (Table 2).
Treatment of Established Bone Metastases
Breast Cancer
Bone is the initial site of relapse in 30% to 40% of women with breast cancer. Although the median survival of breast cancer patients with metastases confined only to the skeleton is longer than those with visceral metastases, the complications associated with bone metastases cause significant morbidity and impair quality of life. Pain develops in up to 75% of breast cancer patients with skeletal metastases, pathologic fracture in 16%, hypercalcemia in 17%, and spinal cord compression in 3%.[32]
Studies demonstrating the beneficial effects of bisphosphonates are described in Table 3.[26,27,33-38] Two double-blind, randomized placebo- controlled trials of IV pamidronate (90 mg) in breast cancer patients who were receiving either chemotherapy or hormonal therapy showed significant reductions in skeletal- related events and pain.[26,33] Among patients receiving chemotherapy, the overall reduction in skeletalrelated events (hypercalcemia, pathologic fracture, spinal cord compression, or radiation for palliation) was 43% vs 56% (P = .008), and the median time to first skeletal event was 14 vs 7 months (P < .001) for the pamidronate- and placebo-treated groups, respectively.[26,38] Among patients receiving hormonal therapy, the overall reduction in skeletal-related events was 56% vs 67% (P = .03), and the median time to first skeletal event was 10 vs 7 months (P = .05) for the pamidronate- and placebotreated groups, respectively.[33] Overall survival was similar in both groups in both of these trials.
TABLE 3

Bisphosphonates in Breast Cancer Patients With Skeletal Metastases
In another double-blind randomized trial, researchers compared zoledronate at 4 or 8 mg IV to pamidronate at 90 mg IV.[27] No statistically significant differences emerged between zoledronate- and pamidronate- treated breast cancer patients in terms of declines in skeletal-related events or time to first skeletal event. Zoledronate was associated with reversible elevations in serum creatinine, primarily at the 8-mg dose and with a 5-minute bolus infusion. With the recommended dose of 4 mg over 15 minutes, the incidence of elevated serum creatinine levels did not differ between zoledronate- and pamidronate- treated patients.[39] The shorter infusion time of zoledronate (15 minutes vs 90 to 120 minutes for pamidronate) and equivalent therapeutic efficacy relative to pamidronate makes zoledronate more convenient for many patients.
• Treatment Controversies-Pamidronate, and more recently zoledronate, are considered part of standard therapy for breast cancer patients with predominantly lytic skeletal metastases. However, several questions remain unanswered.
The optimal duration of bisphosphonate therapy is undefined, but treatment in excess of 2 years appears to be well tolerated.[40] The American Society of Clinical Oncology (ASCO) practice guidelines concluded that once bisphosphonates are initiated, they should be continued until the patient's performance status declines.[ 41] The question of whether every breast cancer patient with skeletal metastases benefits from bisphosphonates also has no firm answers. Many patients have only one or two skeletal metastases and are asymptomatic, or are likely to respond to systemic chemotherapy or hormonal therapy. The ASCO panel concluded that the use of bisphosphonates is a high priority in patients with multiple painful lytic metastases or metastases in weight-bearing bones.
Finally, questions about the costeffectiveness of bisphosphonate added to the cost of breast cancer treatment are relevant, with one analysis concluding that the cost of pamidronate in combination with chemotherapy was $110,000 in the United States and $19,000 in Canada per qualityadjusted life-year gained.[41,42] The former is beyond the range of other accepted medical interventions, whereas the latter is not. This wide disparity was attributed, in part, to the relatively higher cost of pamidronate in the United States.
Prostate Cancer
TABLE 4

Bisphosphonates in Prostate Cancer Patients With Skeletal Complications
Upwards of 85% to 100% of men who die of prostate cancer have skeletal metastases that cause pain, pathologic fracture, and spinal cord compression.[43] Although skeletal metastases from prostate cancer primarily have increased osteoblastic activity, evidence also suggests increased osteoclast activation and bone resorption. This observation is the basis for testing bisphosphonates in these patients. Randomized placebo-controlled trials are described in Table 4.[44-48] Treatment with clodronate or pamidronate failed to produce any reductions in the number of skeletal-related events.[44-47] In contrast, a recently reported randomized, placebo-controlled trial in patients with hormonerefractory metastatic prostate cancer showed a significant reduction in skeletal- related events (44.2% vs 33.2%, P = .021) and an increase in the median time to first skeletal-related event (14+ vs 11 months, P = .011) for zoledronate (4 mg IV).[48] The results of this trial are promising; at this time, however, zoledronate should not be considered standard treatment for skeletal metastases from prostate cancer.[49]
Other Solid Tumors
Zoledronate is also effective in reducing skeletal-related events and increasing the median time to first skeletal event in patients with predominately non-small-cell lung cancer and other nonbreast cancers.[50] In a double-blind placebo-controlled trial of IV zoledronate at 4 mg, skeletal- related events were reduced (37% vs 44%, P = .13), and the median time to first skeletal event was prolonged (8 vs 5 months, P = .02). This trial was the first to show a significant benefit of bisphosphonates in patients with skeletal metastases from solid tumors other than breast and prostate cancer.
Multiple Myeloma
TABLE 5

Bisphosphonates in Multiple Myeloma Patients
Multiple myeloma is a common hematologic malignancy with nearly 15,000 cases reported annually. Nearly 80% of multiple myeloma patients present with lytic skeletal metastases at the time of diagnosis, with pain and pathologic fractures being common features of this disease. Randomizedplacebo controlled trials are outlined in Table 5.[27,51-55] In one trial of oral clodronate,[51] the progression of skeletal metastases was reduced, and in another,[52] the incidence of nonvertebral fractures was reduced. Oral pamidronate, however, did not produce a statistically significant effect on skeletal-related morbidity.[53]
Berenson et al demonstrated the efficacy of IV pamidronate, 90 mg administered every 4 weeks, in patients with advanced multiple myeloma.[ 54] In pamidronate-treated patients, the incidence of skeletal-related events was reduced (28% vs 44%, P = .001), and the median time to first skeletal event was prolonged (21 vs 10 months, P = .008). Although no statistically significant difference in overall survival emerged between the pamidronate- and placebo-treated groups, survival was shown to improve in a planned subset analysis of patients who received second-line chemotherapy. As in breast cancer patients, the benefits of zoledronate and pamidronate in reducing skeletalrelated events were comparable to those seen in multiple myeloma patients.[27]
Prevention of Bone Metastases
TABLE 6

Bisphosphonates in the Prevention of Skeletal Metastases
Results of in vivo studies suggest a potential role for bisphosphonates in preventing skeletal metastases through apoptotic and antitumor effects.[ 21-25] Most of the randomized trials have focused on breast cancer, testing whether bisphosphonates will delay or prevent skeletal metastases. Two types of breast cancer patients have been enrolled in these trials: those at high risk for bone metastases, such as patients with evidence of extraskeletal metastases or presumed breast cancer cells in the bone marrow detected by immunohistochemistry but no radiographic evidence of skeletal metastases; and unselected patients with early-stage breast cancer. The results of these trials are described in Table 6.[56-61]
In a small trial in stage IV breast cancer patients without skeletal metastases, clodronate at 1,600 mg or placebo was administered for 3 years.[56] The investigators reported a significant reduction in the total number of bone metastases (32 vs 63; P < .005). A similar trial of oral pamidronate failed to confirm these results, probably reflecting the poor oral bioavailability of pamidronate.[57]
A larger trial of clodronate at 1,600 mg for 2 years was conducted in breast cancer patients with tumor cells in the bone marrow. At 3 years of follow-up, clodronate-treated patients had significantly fewer skeletal and visceral metastases and an improved overall survival.[58] At 4.5 years of follow-up, the reductions in visceral metastases were no longer significant, but the reductions in skeletal metastases and improvements in survival were maintained.[59] Potential weaknesses of this trial include a highly selected patient population (with tumor cells in the bone marrow identified by immunohistochemistry) and a non-placebo-controlled design.
The results of two randomized trials of adjuvant clodronate in localized, nonmetastatic breast cancer patients are conflicting. Saarto et al reported the results for 299 nodepositive breast cancer patients treated with adjuvant hormonal or chemotherapy with or without clodronate at 1,600 mg/d for 3 years.[60] At the end of 5 years, the incidence of skeletal metastases was equivalent in the clodronate and control groups (21% vs 17%, P = .27). However, clodronate- treated patients had a significantly higher incidence of extraskeletal metastases (43% vs 25%, P = .0007) and a poorer disease-free (56% vs 71%, P = .007) and overall survival (70% vs 83%, P = .009).
Powles et al randomized 1,069 localized breast cancer patients receiving adjuvant tamoxifen, chemotherapy, or both, to either clodronate at 1,600 mg per day for 2 years or placebo.[ 61] At 5.5 years of follow-up, the overall incidence of skeletal metastases (12% vs 15%, P = .127) and extraskeletal metastases (21% vs 24%, P = .257) did not differ. However, in a predetermined subset analysis, skeletal metastases were less frequent (2% vs 5%, P = .016) in the clodronate arm during the 2-year treatment period. Surprisingly, the overall survival benefit just reached statistical significance among clodronate-treated patients (83% vs 79%, P = .047).
The problem of relatively low sample size and the inherent statistical uncertainty in the Saarto trial notwithstanding, it is hard to reconcile the opposite results in these trials. A confirmatory placebo-controlled trial of clodronate (National Surgical Adjuvant Breast and Bowel Project B-34 trial) in localized breast cancer patients is ongoing. At this time, bisphosphonates for the prevention of skeletal metastases should only be offered in the context of a clinical trial.
No published trials have addressed the prevention of skeletal metastases in multiple myeloma patients with osteopenia but without radiographic lytic lesions. However, the ASCO clinical practice guidelines concluded that it is reasonable to recommend bisphosphonates in this patient population.[62]
Bisphosphonates in the Treatment of Osteoporosis in Cancer Patients
Osteoporosis and osteopenia can occur in cancer patients secondary to treatment effects.[63] This is particularly relevant in breast and prostate cancers due to the prevalence of these diseases.
Premenopausal Women
Chemotherapy-induced ovarian failure is common among premenopausal breast cancer patients, with prospective studies documenting rapid and accelerated loss of bone mineral density.[64] Three randomized controlled trials have shown that oral clodronate and risedronate (Actonel) decrease bone loss in premenopausal women receiving adjuvant chemotherapy.[ 65-67]
The Cancer and Leukemia Group B is currently performing a randomized trial of zoledronate in premenopausal women receiving adjuvant chemotherapy. In addition to adequate intake of vitamin D and calcium, weight-bearing exercise, and counseling about the relationship between bone loss and alcohol and cigarette smoking, breast cancer patients who develop ovarian failure should have their bone density measured and, if appropriate, should be treated with bisphosphonates approved for the prevention and treatment of osteoporosis.[68]
Postmenopausal Women
In postmenopusal breast cancer patients, the aromatase inhibitors act by inhibiting the aromatase enzyme responsible for estrogen production. Two such drugs, anastrozole (Arimidex) and letrozole (Femara), are superior to tamoxifen in the treatment of postmenopausal metastatic breast cancer patients with estrogen-receptor- positive tumors and are approved for this indication. A recent large study demonstrated the superiority of anastrozole over tamoxifen in postmenopausal breast cancer patients with localized disease and led to its approval for this indication.[69] One troubling side effect of anastrozole is skeletal fractures, which develop more frequently with longer follow-up in patients taking the drug. As in premenopausal breast cancer patients with ovarian failure, it is reasonable for patients taking this drug to get their bone density checked and, if appropriate, initiate bisphosphonate therapy. Ongoing randomized trials are evaluating aromatase inhibitors in combination with bisphosphonates in postmenopausal women with early-stage, estrogen-receptor- positive breast cancer.
Men
TABLE 7

Indications for Bisphosphonates in Cancer Patients
Men with prostate cancer who are castrated or receive gonadotropinreleasing hormone agonists lose bone and are at risk of osteoporosis.[70] In a small-randomized study, prostate cancer patients were treated with these agonists, with or without pamidronate.[ 71] The pamidronate-treated patients did not lose bone mineral density, whereas patients treated with the gonadotropin-releasing hormone agonist alone experienced significant bone loss. Awareness of bone loss is particularly important for prostate cancer patients who are receiving these agonists in the adjuvant setting. It is reasonable to measure bone density in these patients as well.
Summary
The current indications for bisphosphonate use in cancer patients are described in Table 7. For skeletal metastases in patients with breast cancer, multiple myeloma, or other solid tumors, bisphosphonates are important adjuncts to systemic therapy. Pamidronate at 90 mg infused over 90 to 120 minutes is comparable in efficacy and side effects to zoledronate at 4 mg infused over 15 minutes.
REFERENCE GUIDE
Therapeutic Agents
Mentioned in This Article
Anastrozole (Arimidex)
Clodronate
Letrozole (Femara)
Pamidronate
Risedronate (Actonel)
Tamoxifen
Zoledronate
(zoledronic acid, Zometa)
Brand names are listed in parentheses only if a drug is not available generically and is marketed as no more than two trademarked or registered products. More familiar alternative generic designations may also be included parenthetically.
For prostate cancer patients with skeletal metastases, the results with zoledronate are promising, but additional trials are needed before bisphosphonates become part of standard treatment for metastatic prostate cancer. The available trials on the use of bisphosphonates for the prevention of skeletal metastases in breast cancer patients have demonstrated mixed results. However, all the trials used clodronate, a less potent oral bisphosphonate. Ongoing trials are evaluating the preventive role of the third-generation bisphosphonates in breast cancer patients. Until the results of these trials are presented, bisphosphonates should only become a component of adjuvant treatment in the context of a clinical trial. Finally, bone loss is a common consequence of cancer treatment, and awareness of this complication is essential. Bone loss can be treated with the usual measures indicated for the management of osteoporosis, including bisphosphonates.
What does the future hold? Bisphosphonates have direct antitumor and antiangiogenic effects, and several trials in breast and multiple my myeloma patients have shown improved survival among bisphosphonatetreated patients. Dedicated randomized trials to evaluate the antitumor effects of bisphosphonates in combination with chemotherapy are warranted. Preclinical and recent studies in postmenopausal, osteoporitic patients and patients with breast cancer and multiple myeloma show promising results for recombinant osteoprotegerin as an inhibitor of bone resorption.[72,73] Parathyroid hormone- releasing protein is a potential target for cancer therapy in preclinical studies.[74] Future studies of these novel agents in combination with bisphosphonates may prove them to be useful in the treatment and prevention of skeletal metastases.
Financial Disclosure:The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.
Shapiro CL: Bisphosphonates in breastcancer patients with skeletal metastases. HematolOncol Clin North Am 8:153-163, 1994.
2.
Lories RJ, Luyten FP: Osteoprotegerinand osteoprotegerin-ligand balance: A newparadigm in bone metabolism providing newtherapeutic targets. Clin Rheumatol 20:3-9,2001.
3.
Hofbauer LC, Neubader A, HeufelderAE: Receptor activator of nuclear factor-kbligand and osteoprotegerin. Cancer 92:460-70, 2001.
4.
Powell GJ, Southby J, Danks JA, et al:Localization of parathyroid hormone-relatedprotein in breast cancer metastases-increasedincidence in bone compared with other sites.Cancer Res 51:3059, 1991.
5.
Guise TA: Molecular mechanisms of osteolyticbone metastases. Cancer 88(suppl12):2892-2898, 2000.
6.
Mundy GR, Toshiyuki Y: Bisphosphonatesas anticancer drugs. N Engl J Med339:398-400, 1998.
7.
Yasuda H, Shima N, Nakagawa N, et al:Identity of osteoclastogenesis inhibitory factor(OCIF) and osteoprotegerin (OPG): Amechanism by which OPG/OCIF inhibits osteoclastogenesisin vitro. Endocrinology139:1329-1337, 1998.
8.
Clohisy DR, Ramnaraine ML, Scully S,et al: Osteoprotegerin inhibits tumor-inducedosteoclastogenesis and bone tumor growth inosteopetrotic mice. J Orthop Res 18:967-976,2000.
9.
Goltzman D, Karaplis AC, Kremer R, etal: Molecular basis of the spectrum of skeletalcomplications of neoplasia. Cancer 88:2903-2908, 2000.
10.
Koutsilieris M, Frenette G, Lazure C,et al: Urokinase-type plasminogen activator:A paracrine factor regulating the bioavailabilityof IGFs in PA-III cell-induced osteoblasticmetastases. Anticancer Res 13:481-486,1993.
11.
Cohen P, Graves HC, Peehl DM, et al:Prostate-specific antigen (PSA) is an insulinlikegrowth factor binding protein-3 (IGFBP-3) protease found in seminal plasma. J ClinEndocrinol Metab 75:1046-1053, 1992.
12.
Van der Pluijm G, Vloedgraven H, vanBeek E, et al: Bisphosphonates inhibit theadhesion of breast cancer cells to bone matricesin vitro. J Clin Invest 98:698-705, 1996.
13.
Boissier S, Magneto S, Frappart L, et al:Bisphosphonates inhibit prostate and breastcarcinoma cell adhesion to unmineralized andmineralized bone extracellular matrices. CancerRes 57:3890-3894, 1997.
14.
David P, Hguyen H, Barbier A, et al:The bisphosphonate tiludronate is a potent inhibitorof the osteoclast vacuolar H(+)-ATPase.J Bone Miner Res 11:1498-1507, 1996.
15.
Rogers MJ, Gordon S, Benford HL, etal: Cellular and molecular mechanisms of actionof bisphosphonates. Cancer 88:2961-2978,2000.
16.
Senaratne SG, Pirianov G, Mansi JL, etal: Bisphosphonates induce apoptosis in humanbreast cancer cells. Br J Cancer 82:1459-1468, 2000.
17.
Shipman CM, Rogers MJ, Apperley JF,et al: Bisphosphonates induce apoptosis in humanmyeloma cell lines: A novel anti-tumouractivity. Br J Haematol 98:665-672, 1997.
18.
Hughes DE, MacDonald BR, RussellRG, et al: Inhibition of osteoclast-like cellformation by bisphosphonates in long-termcultures of human bone marrow. J Clin Invest83:1930-1935, 1989.
19.
Teronen O, Heikkila P, Konttinen YJ, etal: MMP downregulation and inhibition bybisphosphonates. Ann N Y Acad Sci 878:453-465, 1999.
20.
Boissier S, Ferreras M, Peyruchaud O,et al: Bisphosphonates inhibit breast and prostatecarcinoma cell invasion, an early event inthe formation of bone metastases. Cancer Res60:2949-2954, 2000.
21.
Lee MV, Fong EM, Singer FR, et al:Bisphosphonate treatment inhibits growth ofprostate cancer cells. Cancer Res 61:2602-2608, 2001.
22.
Fromigue O, Lagneaux L, Body JJ: Bisphosphonatesinduce breast cancer cell deathin vitro. J Bone Miner Res 15:2211-2221, 2000.
23.
Green J, Gschaidmeier H, Yoneda T, etal: Zoledronic acid potently inhibits tumorinducedosteolysis in two animal models ofbreast cancer metastatic to bone. Ann Oncol11(suppl 4):14, 2000.
24.
Nobuyuki H, Hiraga T, Williams PJ, etal: The bisphosphonate zoledronic acid inhibitsmetastases to the bone and liver with suppressionof osteopontin production in mousemammary tumor. J Bone Miner Res 16(suppl1):S191, 2001.
25.
Green JR: Antitumor effects of bisphosphonates.Cancer 97(3 suppl):840-847, 2003.
26.
Hortobagyi GN, Theriault RL, Porter L,et al: Efficacy of pamidronate in reducing skeletalcomplications of breast cancer and lyticbone metastases. N Engl J Med 335:1785-1791, 1996.
27.
Rosen LS, Gordon D, Kaminski M, etal: Zoledronic acid versus pamidronate in thetreatment of skeletal metastases in patientswith breast cancer or osteolytic lesions of multiplemyeloma: A phase III, double blind, comparativetrial. Cancer J 7:377-387, 2001.
28.
Bajorunas DR: Clinical manifestationsof cancer-related hypercalcemia. Semin Oncol17:16-25, 1990.
29.
Body JJ, Dumon JC: Treatment of tumor-induced hypercalcemia with the bisphosphonatepamidronate: Dose-responserelationship and influence of tumor type. AnnOncol 5:359-363, 1994.
30.
Nussbaum SR, Younger J, VandepolCJ, et al: Single-dose intravenous therapy withpamidronate for the treatment of hypercalcemiaof malignancy: Comparision of 30-, 60-,90-mg doses. Am J Med 95:297-304, 1993.
31.
Major P, Lortholary A, Hon J, et al:Zoledronic acid is superior to pamidronate inthe treatment of hypercalcemia of malignancy:A pooled analysis of two randomized, controlledclinical trials. J Clin Oncol 19:558-567,2001.
32.
Coleman RE: Skeletal complications ofmalignancy. Cancer 80(8 suppl):1588-1594,1997.
33.
Theriault RL, Lipton A, Hortobagyi GN,et al: Pamidronate reduces skeletal morbidityin women with advanced breast cancer andlytic bone lesions: A randomized, placebocontrolledtrial. J Clin Oncol 17:846-854, 1999.
34.
Elomaa I, Blomqvist C, Porkka L, et al:Treatment of skeletal disease in breast cancer:A controlled clodronate trial. Bone 8(suppl1):53-56, 1987.
35.
Paterson AH, Powles TJ, Kanis JA, etal: Double-blind controlled trial of oral clodronatein patients with bone metastases frombreast cancer. J Clin Oncol 11:59-65, 1993.
36.
Van Holten-Verzantvoort AT, KroonHM, Bijvoet OL, et al: Palliative pamidronatetreatment in patients with bone metastases frombreast cancer. J Clin Oncol 11:491-498, 1993.
37.
Conte PF, Latreille J, Mauriac L, et al:Delay in progression of bone metastases inbreast cancer patients treated with intravenouspamidronate: Results from a multinational randomizedcontrolled trial. J Clin Oncol 14:2552-2559, 1996N
38.
Hortobagyi GN, Theriault RL, LiptonA, et al: Long-term prevention of skeletal complicationsof metastatic breast cancer with pamidronate.Protocol 19 Aredia Breast CancerStudy Group. J Clin Oncol 16:2038-2044,1998.
39.
Cohen MH, Dagher R, Griebel DJ, et al:US Food and Drug Administration Drug approvalsummaries: Imatinib mesylate, mesnatablets, and zoledronic acid. Oncologist 7:393-400, 2002.
40.
Ali SM, Esteva FJ, Hortobagyi GN, etal: Safety and efficacy of bisphosphonates beyond24 months in cancer patients. J ClinOncol 19:3434-3437, 2001.
41.
Hillner BE, Ingle JN, Berenson JR, etal: American Society of Clinical Oncologyguideline on the role of bisphosphonates inbreast cancer. J Clin Oncol 18:1378-1391,2000.
42.
Hillner BE, Weeks JC, Desch CE, et al:Pamidronate in prevention of bone complicationsin metastatic breast cancer: A cost-effectivenessanalysis. J Clin Oncol 18:72-79, 2000.
43.
Carlin BI, Andriole GL: The naturalhistory, skeletal complications, and managementof bone metastases in patients with prostatecarcinoma cancer. Cancer 88:2989-2994,2000.
44.
Elomaa I, Kylmala T, Tammela T, et al:Effect of oral clodronate on bone pain. IntUrol Nephrol 24:159-166, 1992.
45.
Kylmala T, Taube T, Tammela TL, etal: Concomittant IV and oral clodronate in therelief of bone pain-A double-blind placebocontrolledstudy in patients with prostate cancer.Br J Cancer 76:939-942, 1997.
46.
Ernst DS, Tannock IF, Venner PM, etal: Randomized, placebo-controlled trial ofmitoxantrone/prednisone and clodronate versusmitoxantrone/prednisone alone in patientswith hormone-refractory prostate cancer(HRPC) and pain: National Cancer Institute ofCanada clinical trials group study (abstract705). Proc Am Soc Clin Oncol 21:177a, 2002.
47.
Lipton A, Small E, Saad F, et al: Thenew bisphosphonate, zometa decreases skeletalcomplications in both lytic and blastic lesions:A comparision to pamidronate (abstract).Cancer Invest 20:45-47, 2001.
48.
Saad F, Gleason DM, Murray R, et al: Arandomized, placebo-controlled trial ofzoledronic acid in patients with hormone-refractorymetastatic prostate carcinoma. J NatlCancer Inst 94:1458-1468, 2002.
49.
Canil CM, Tannock IF: Should bisphosphonatesbe used routinely in patients withprostate cancer metastatic to bone? J Natl CancerInst 94:1422-1423, 2002.
50.
Rosen L, Gordon D, Tchekmedyian S,et al: Zoledronic acid significantly reducesskeletal related events in patients with bonemetastases from solid tumors (abstract 1179).Proc Am Soc Clin Oncol 21:295a, 2002.
51.
McCloskey EV, MacLennan IC, DraysonMT, et al: A randomized trial of the effectsof clodronate on skeletal morbidity inmultiple myeloma. MRC Working Party onLeukemia in Adults. Br J Haematol 100:317-325, 1998.
52.
Lahtinen R, Laakso M, Palva I, et al:Randomized, placebo-controlled multicentertrial of clodronate in multiple myeloma. FinnishLeukemia Group. Lancet 340:1049-1052,1992.
53.
Brincker H, Westin J, Abildgaard N, etal: Failure of oral pamidronate to reduce skeletalmorbidity in multiple myeloma: A doubleblindplacebo-controlled trial. Danish-Swedishco-operative group. Br J Haematol 101:280-286, 1998.
54.
Berenson JR, Lichtenstein A, Porter L,et al: Long-term pamidronate treatment of advancedmultiple myeloma patients reducesskeletal events. Myeloma Aredia Study Group.J Clin Oncol 6:593-602, 1998.
55.
Mennsen HD, Sakalova A, Fontana A,et al: Effects of long-term intravenous ibandronatetherapy on skeletal-related events, survival,and bone resorption markers in patientswith advanced multiple myeloma. J Clin Oncol20:2353-2359, 2002.
56.
Kanis JA, Powles T, Paterson AH, et al:Clodronate decreases the frequency of skeletalmetastases in women with breast cancer.Bone 19:663-667, 1996.
57.
Ford JM, van Oosterom, Brincker H, etal: Oral pamidronate: Negative results fromthree double-blind, placebo controlled trialsin hypercalcemia, myeloma, and the preventionof bone metastases (abstract). Bone 22(suppl3):58S, 1998.
58.
Diel IJ, Solomayer EF, Costa SD, et al:Reduction in new metastases in breast cancerwith adjuvant clodronate treatment. N Engl JMed 339:357-363, 1998.
59.
Deil IJ, Solomayer EF, Gollan C, et al:Bisphosphonates in the reduction of metastasesin breast cancer-results of the extended follow-up of the study population (abstract 314).Proc Am Soc Clin Oncol 19:82a, 2000.
60.
Saarto T, Blomqvist C, Virkkunen P etal: Adjuvant clodronate treatment does notreduce the frequency of skeletal metastases innode-positive breast cancer patients: 5-yearresults of a randomized controlled trial. J ClinOncol 19:10-17, 1996.
61.
Powles T, Paterson S, Kanis JA, et al:Randomized, placebo-controlled trial of clodronatein patients with primary operable breastcancer. J Clin Oncol 20:3219-3224, 2002.
62.
Berenson J, Hillner BE, Kyle RA, et al:American Society of Clinical Oncology practiceguidelines: The role of bisphosphonatesin multiple myeloma. J Clin Oncol 20:3719-3736, 2002.
63.
Pfeilschifter J, Diel IJ: Osteoporosisdue to cancer treatment: Pathogenesis andmanagement. J Clin Oncol 18:1570-1593,2000.
64.
Shapiro CL, Manola J, Leboff M: Ovarianfailure after adjuvant chemotherapy is associatedwith rapid bone loss in women withearly-stage breast cancer. J Clin Oncol19:3303-3305, 2001.
65.
Delmas PD, Balena R, Confravreux E,et al: Bisphosphonate risendronate preventsbone loss in women with artificial menopausedue to chemotherapy of breast cancer: A double-blind, placebo-controlled study. J ClinOncol 15:955-962, 1997.
66.
Vehmanen L, Saarto T, Elomaa I, et al:Long-term impact of chemotherapy-inducedovarian failure on bone mineral density (BMD)in premenopausal breast cancer patients. Theeffect of adjuvant clodronate treatment. Eur JCancer 37:2373-2378, 2001.
67.
Saarto T, Blomqvist C, Valimaki M, etal: Chemical castration induced by adjuvantcyclophosphamide, methotrexate, and fluorouracilchemotherapy causes rapid bone loss thatis reduced by clodronate: A randomized studyin premenopausal breast cancer patients. J ClinOncol 15:1341-1347, 1997.
68.
Watts NB: Treatment of osteoporosiswith bisphosphonates. Endocrinol Metab ClinNorth Am 27:419-439,1998.
69.
The ATAC Trialists’ group: Anastrozolealone or in combination with tamoxifenversus tamoxifen alone for adjuvant treatmentof postmenopausal women with early breastcancer: First results of the ATAC randomizedtrial. Lancet 359:2131-2139, 2002.
70.
Mittan D, Lee S, Miller E, et al: Boneloss following hypogonadism in men withprostate cancer treated with GnRH analogs.J Clin Endocrinol Metab 87:3656-3661,2002.
71.
Smith MR, McGovern FJ, Zietman AL,et al: Pamidronate to prevent bone loss duringandrogen deprivation therapy for prostate cancer.N Engl J Med 345:948-955, 2001.
72.
Body JJ, Greipp P, Coleman RE, et al:A phase I study of AMGN-0007, a recombinantosteoprotegerin construct, in patients withmultiple myeloma or breast carcinoma relatedbone metastases. Cancer 97(3 suppl):887-892,2003.
73.
Croucher PI, Shipman CM, Van CampBV, et al: Bisphosphonates and osteoprotegerinas inhibitors of myeloma bone disease. Cancer97(3 suppl):818-824, 2003.
74.
Ogata E: Parathyroid hormone-relatedprotein as a potential target of therapy forcancer-associated morbidity. Cancer 88(12suppl):2909-2911, 2000.