Irinotecan and Paclitaxel in Metastatic Adenocarcinoma of the Esophagus and Gastric Cardia
Both irinotecan (CPT-11, Camptosar) and paclitaxel have beenshown to have single-agent activity in adenocarcinomas of the esophagusand gastric cardia. A phase I trial of the combination at UCLAestablished the dose as irinotecan at 225 mg/m2 and paclitaxel at100 mg/m2 every 3 weeks. Preliminary data from a phase II trial of thisregimen in adenocarcinomas of the gastroesophageal junction showgood tolerability and promising activity (response rate of 27%), even inA previously treated patients.
ABSTRACT: Both irinotecan (CPT-11, Camptosar) and paclitaxel have beenshown to have single-agent activity in adenocarcinomas of the esophagusand gastric cardia. A phase I trial of the combination at UCLAestablished the dose as irinotecan at 225 mg/m2 and paclitaxel at100 mg/m2 every 3 weeks. Preliminary data from a phase II trial of thisregimen in adenocarcinomas of the gastroesophageal junction showgood tolerability and promising activity (response rate of 27%), even inA previously treated patients.
A previously treated patients. denocarcinomas of the gastroesophagealjunction compriseadenocarcinomas of theesophagus and gastric cardia. The cancersare quite similar histologicallyand epidemiologically, and they aredifferent from either squamous cellcarcinoma of the esophagus or themore frequent distal adenocarcinomaof the stomach. The etiology of thesemalignancies remains obscure, butthey appear to be related to chronicgastroesophageal reflux and intestinalmetaplasia.[1] The epidemiologyand natural history of adenocarcinomasof the gastric cardia appear to bemuch more similar to that of adenocarcinomaof the esophagus than thatof the distal stomach. In large tumorsof the gastroesophageal junction, it isdifficult to distinguish between esophagealand gastric origin.Once quite rare, these cancers arethe most rapidly increasing solid tumorsin the United States and WesternEurope,[2] and now account formore than 50% of esophageal carcinomasand an increasing number ofgastric malignancies. Unfortunately,most patients' cancers are not resectableat the time of diagnosis or relapseafter surgery. The standard ofcare has historically consisted of combinationsof fluorouracil (5-FU) andcisplatin.[3,4] Although response ratesare reasonable in the 20% to 30%range survival remains relatively limited(6 to 11 months), and the toxicityof treatment, particularly from the cisplatin,can be severe in these fragilepatients.The limitations of standard therapyhas led to the study of newer agentsin adenocarcinomas of the gastroesophagealjunction, including taxanesand topoisomerase I inhibitors. Paclitaxelmay be the most active singleagent in gastroesophageal malignancies,with Ajani et al reporting a responserate of 34% in adenocarcinomaof the esophagus.[5] Combinations ofpaclitaxel with cisplatin and 5-FUhave also resulted in significant response rates of 48% to 51%.[6,7] Thetopoisomerase I inhibitor irinotecan(CPT-11, Camptosar) possesses activityin a number of adenocarcinomas,including those of the colon andlung. Single-agent response rates of14% in adenocarcinoma of the esophagus[8] and 15% in esophageal andgastric carcinomas[9] have lead it tobe used in combination with 5-FU/leucovorin[10] and cisplatin,[11]where respective response rates of22% in gastric adenocarcinoma and57% in advanced esophageal cancer(52% adenocarcinoma, 66% squamouscarcinoma) were reported.The mostly nonoverlapping toxicitiesand different mechanisms of actionof irinotecan and paclitaxel led tothe examination of this combinationin a phase I setting by Rosen et al.[12]No significant drug interactions wereseen and the combination was quitetolerable, with neutropenia being thedose-limiting toxicity with some activityseen in upper gastrointestinaltract cancers. Therefore, the regimenwas taken forward into a phase II trialin adenocarcinomas of the gastroesophagealjunction.
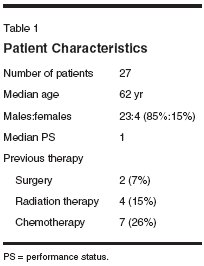
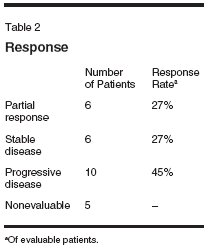
Study Design andPatient PopulationThe protocol was approved by theUCLA, OHSU, Northwestern University,and USC institutional reviewboards. The study was a single-armmulticenter phase II study. The primaryend point was response rate andsecondary end points were safety andsurvival. A two-stage Simon designwas used to calculate sample size.[13]If one response in the first 13 patientswas seen then the study was to accruean additional 14 patients. Patients withhistologically proven adenocarcinomaof the esophagus or gastric cardiawere eligible for the trial. Documentationof the location of the tumor byendoscopy or radiologic study wasrequired. The patients had to have bidimensionallymeasurable disease andadequate hematologic, liver, and renalfunction. Patients required an EasternCooperative Oncology Groupperformance status of > 2. Patientswho had received more than one priortreatment regimen for metastatic disease,were receiving antiepilepticmedication, and had known Gilbert'ssyndrome were excluded. Patients hadscreening laboratories, computed tomography(CT) of the chest, abdomen,and pelvis, and a history andphysical prior to the start of treatment.MethodsPatients received pretreatment withdexamethasone at 20 mg IV, diphenhydramineat 50 mg IV, famotidine at20 mg IV (or similar H2 receptor antagonist),and granisetron (Kytril) at1 mg IV (or similar 5HT3 antagonist).After pretreatment, patients were treatedwith irinotecan at 225 mg/m2 over90 minutes followed by paclitaxel at100 mg/m2 over 3 hours every 3weeks. Atropine was administered asneeded for cholinergic symptoms. Patientswere counseled on the use of,and provided with, high-dose loperamidein case of diarrhea.Response was determined everythree cycles (9 weeks) using CT ormagnetic resonance imaging (MRI)scans using the World Health Organizationcriteria. Responses had to beverified on another radiologic study atleast 4 weeks later, though there wasno provision for early scans as part ofthe protocol. Toxicity was graded usingthe National Cancer Institute CommonToxicity Criteria version 2.0.ResultsA total of 27 patients were enrolledonto the study; see Table 1 forthe demographics. Median age was62 years (range: 45-82 years). Thestriking male predominance (85%) isconsistent with the epidemiology ofadenocarcinomas of the gastroesophagealjunction. Eight patients had priortreatment for their cancer,including some receiving more thanone treatment modality. At this time,22 patients are evaluable for response.The median number of treatment cycleswas 3 (range: 3-20 cycles) andmedian survival was 8.6 months(range: 2-24+ months). The documentedpartial response rate was 27%,with 27% of patients having stabledisease (Table 2). Half of the pretreatedpatients were evaluable hadpartial responses (3 of 6).Toxicity was manageable, withthree admissions for neutropenic fevers.In the 16 patients with completetoxicity data representing 110 cycles,only nine grade 3 and one grade 4neutropenia were reported. Diarrheawas uncommon with only three reportsof grade 3 and none of grade 4.DiscussionThe proper treatment of advancedcarcinoma of the gastroesophagealjunction remains unclear. Clearly,irinotecan and paclitaxel are both activeagents in this disease, and theircombination appears to have significantactivity, even in previously treatedpatients. While single-agentpaclitaxel possesses activity, this is atthe cost of significant neutropenia thatmay require the use of growth factors.[5] Other combinations usingthese drugs may have gastrointestinaland bone marrow toxicity.[7] This regimenis convenient and well toleratedwith minimal neutropenia and diarrhea.In addition to collecting clinicaldata, we plan to examine molecular correlates including UDP-glucuronosyltransferase(UGT) 1A1 polymorphisms[14] and beta-tubulin IIIisoform levels[15] potentially to helpidentify, respectively, both patients atincreased risk of toxicity, and tumorsless likely to respond to taxanes.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.
Avidan B, Sonnenberg A, Schnell TG, etal: Hiatal hernia size, Barrett’s length, and severityof acid reflux are all risk factors foresophageal adenocarcinoma. Am J Gastroenterol97:1930-1936, 2002.
2.
Blot WJ: Esophageal cancer trends andrisk factors. Semin Oncol 21:403-410, 1994.
3.
Fearon E: Cancer of the esophagus, In:DeVita VT, Jr, Hellman S, Rosenberg SA (eds):Cancer: Principles and Practice of Oncology(6th ed), pp 1051-1091. Philadelphia, LippincottWilliams & Wilkins, 2001.
4.
Ajani JA: Current status of therapy foradvanced gastric carcinoma. Oncology 12(2suppl 6):99-102, 1998.
5.
Ajani JA, Ilson DH, Daugherty K, et al:Activity of Taxol in patients with squamous cellcarcinoma and adenocarcinoma of the esophagus.J Natl Cancer Inst 86:1086-1091, 1994.
6.
Kim YH, Shim SW, Kim BS: Paclitaxel,5-fluorouracil, and cisplatin combination chemotherapyfor the treatment of advanced gastriccarcinoma. Cancer 85:295-301, 1999.
7.
Ilson DH, Ajani J, Bhalla K, et al: Phase IItrial of paclitaxel, fluorouracil, and cisplatin inpatients with advanced carcinoma of the esophagus.J Clin Oncol 16:1826-1834, 1998.
8.
Hecht JR, Parson M, Rosen L: A phase IItrial of irinotecan (CPT-11) in patients withadenocarcinoma of the esophagus and gastriccardia (abstract 1100). Proc Am Soc Clin Onocol18:287a, 1999.
9.
Enzinger P, Kulke M, Clark J, et al: PhaseII trial of CPT-11 in previously untreated patientswith advanced adenocarcinoma of theesophagus and stomach (abstract 1243). ProcAm Soc Clin Oncol 19:315a, 2000.
10.
Blanke CD, Haller DG, Benson AB, etal: A phase II study of irinotecan with 5-fluorouraciland leucovorin in patients with previouslyuntreated gastric adenocarcinoma. AnnOncol 12:1575-80, 2001.
11.
Ilson DH, Saltz L, Enzinger P, et al:Phase II trial of weekly irinotecan plus cisplatinin advanced esophageal cancer. J Clin Oncol17:3270-5, 1999.
12.
Rosen P, Schaaf LJ, Knuth DW, et al:Phase I and pharmacokinetic trial of CPT-11and paclitaxel in advanced cancers (abstract679). Proc Am Soc Clin Oncol 18:177a, 1999.
13.
Simon R: Optimal two-stage designs forphase II clinical trials. Control Clin Trials 10:1-10, 1989.
14.
Ando Y, Saka H, Ando M, et al: Polymorphismsof UDP-glucuronosyltransferasegene and irinotecan toxicity: A pharmacogeneticanalysis. Cancer Res 60:6921-6926, 2000.
15.
Banerjee A: Increased levels of tyrosinatedalpha-, beta(III)-, and beta(IV)-tubulinisotypes in paclitaxel-resistant MCF-7 breastcancer cells. Biochem Biophys Res Commun293:598-601, 2002.