Improving the Toxicity of Irinotecan/5-FU/ Leucovorin: A 21-Day Schedule
Irinotecan (CPT-11, Camptosar) is one of the new generation ofchemotherapeutic agents that has activity in advanced colorectal cancer.It has antitumor efficacy as a single agent, and also has beencombined with fluorouracil (5-FU) and leucovorin (IFL) to treat thesepatients. Randomized studies have confirmed the superiority of IFL to5-FU and leucovorin alone with regard to patient survival, time toprogression, and tumor response rate. The optimal schedule for combiningthese agents remains uncertain, but in the United States, theschedule of IFL weekly for 4 consecutive weeks repeated every 6 weeks,according to the schedule reported by Saltz et al, has been widely used,although with some toxicity (especially myelosuppression and diarrhea).In an attempt to improve the tolerability of IFL, some haveadvocated modifying the schedule of IFL to weekly for 2 weeks, withrepeated cycles every 21 days. Twenty-three patients with advancedcolorectal cancer have been treated on this schedule at a single institution.Therapy was well tolerated, with 35% of patients experiencinggrade 3/4 neutropenia, two of whom had episodes of febrile neutropenia,and 9% with grade 3/4 diarrhea. The median relative dose intensityof irinotecan administered in the first 18 patients treated with thisregimen was 94%. These data support the hypothesis that modifying theschedule of administration of IFL improves the tolerability and abilityto deliver the regimen, but must be confirmed by randomized prospectivestudies, which may also attempt to evaluate the role of bolus 5-FUin the treatment of advanced colorectal cancer.
ABSTRACT: Irinotecan (CPT-11, Camptosar) is one of the new generation ofchemotherapeutic agents that has activity in advanced colorectal cancer.It has antitumor efficacy as a single agent, and also has beencombined with fluorouracil (5-FU) and leucovorin (IFL) to treat thesepatients. Randomized studies have confirmed the superiority of IFL to5-FU and leucovorin alone with regard to patient survival, time toprogression, and tumor response rate. The optimal schedule for combiningthese agents remains uncertain, but in the United States, theschedule of IFL weekly for 4 consecutive weeks repeated every 6 weeks,according to the schedule reported by Saltz et al, has been widely used,although with some toxicity (especially myelosuppression and diarrhea).In an attempt to improve the tolerability of IFL, some haveadvocated modifying the schedule of IFL to weekly for 2 weeks, withrepeated cycles every 21 days. Twenty-three patients with advancedcolorectal cancer have been treated on this schedule at a single institution.Therapy was well tolerated, with 35% of patients experiencinggrade 3/4 neutropenia, two of whom had episodes of febrile neutropenia,and 9% with grade 3/4 diarrhea. The median relative dose intensityof irinotecan administered in the first 18 patients treated with thisregimen was 94%. These data support the hypothesis that modifying theschedule of administration of IFL improves the tolerability and abilityto deliver the regimen, but must be confirmed by randomized prospectivestudies, which may also attempt to evaluate the role of bolus 5-FUin the treatment of advanced colorectal cancer.
Irinotecan (CPT-11, Camptosar) isa semisynthetic derivative ofcamptothecin sodium, which itselfis the active extract from the bark ofthe Chinese/Tibetan deciduous treeCamptotheca acuminata (Nyssaceaefamily). Although early developmentof this camptothecin was stymied bythe toxicity of the compound, in particularmyelosuppression and hemorrhagiccystitis, irinotecan has beenmuch better tolerated, with the primarytoxicities being myelosuppressionand diarrhea.[1] Subsequent studiesin humans have demonstrated thatirinotecan has activity in a number ofmalignancies, including colorectal,[2,3] gastroesophageal,[4-6] pancreatic,[7] lung,[8,9] breast,[10] andgynecologic cancers.[11,12] In theUnite States, irinotecan is currentlyindicated for use in patients with advancedcolorectal cancer.In 1998, two studies proved thebenefit of irinotecan in patients withadvanced colorectal cancer that hadprogressed despite prior therapy withthe then-standard therapy, fluorouracil(5-FU). Cunningham et al[13] reportedthat salvage irinotecan (n = 189) at300 to 350 mg/m2 intravenously (IV)every 3 weeks significantly increased1-year survival in comparison to supportivecare alone (n = 90): 36.2%compared to 13.8%. In the same patientpopulation, Rougier et al[14] randomized267 patients to 300 mg/m2of irinotecan every 3 weeks or infusional5-FU. Again, the 1-year andmedian survivals were increased inpatients treated with irinotecan(n = 133), at 45% and 10.8 months vs32% and 8.5 months, respectively.[14]Other studies confirmed that irinotecanhas antitumor activity in patientswithout previous chemotherapy for metastaticcolorectal cancer.[2,3,15,16] Asa result, irinotecan has become widelyaccepted for use in patients withmetastatic colorectal cancer.
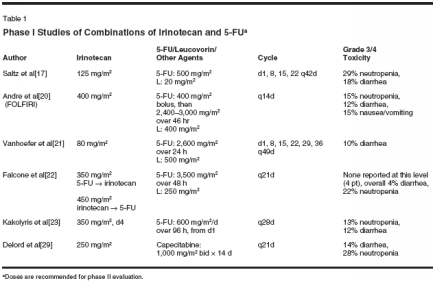
Irinotecan and 5-FUBased on the differing mechanismsof activity of the two most active antineoplasticagents in colorectal and othergastrointestinal cancers, thesedrugs-irinotecan and 5-FU-havebeen administered together. A numberof methods of combining irinotecanand 5-FU with leucovorin (IFL)have been evaluated, but the optimalcombination and schedule remain uncertain(Table 1). Given the multitudeof 5-FU treatment schedules usedaround the world, this is not surprising.However, the most widely usedcombinations of irinotecan and 5-FUare based upon a bolus administrationof therapy.In a phase I study, Saltz et al[17]combined irinotecan, 5-FU, and leucovorin in a weekly for 4 weeks schedule,with cycles repeated every 6weeks (therapy administered on days1, 8, 15, and 22 every 42 days). Sequentialescalations of 5-FU, thenirinotecan, were performed, and thedoses recommended for further evaluationwere 125 mg/m2 of irinotecaninfused IV over 90 minutes, 500 mg/m2 of 5-FU by bolus, and 20 mg/m2 ofleucovorin by IV bolus. The primarydose-limiting toxicity was neutropenia,although diarrhea was common.[17]Furthering the evaluation of thispromising combination, Saltz et al[18]reported a study of 683 patients whowere randomized to either weekly IFLwith this schedule (n = 231), 5-FUand leucovorin on the Mayo clinicschedule (n = 226), or irinotecan at125 mg/m2 IV for 4 consecutive weeks(n = 226), again followed by a 2-weekbreak. This study demonstrated a significantsuperiority of IFL in medianprogression-free survival, objectiveresponse rate, and median survival. Inparticular, therapy with IFL resultedin a 36% decrease in risk of progression, and a 22% decrease in risk ofdeath in comparison to the previousstandard therapy, 5-FU and leucovorin.[18]The so-called de Gramont regimenhas been an accepted standard combinationof 5-FU and leucovorin for advancedcolorectal cancer inFrance.[19] A simplified version ofthis regimen, with the 5-FU administeredas a 400 mg/m2 IV bolus, followedby a 46-hour continuousinfusion at 2,400 to 3,000 mg/m2, every2 weeks, has been combined with180 mg/m2 irinotecan on the first dayof therapy (FOLFIRI), in a study reportedby Andre et al.[20] As salvagetherapy, limited antitumor activity wasnoted. In 33 treated patients, two (6%)patients had partial responses and 20experienced stabilization of disease.Therapy was well tolerated, with 15%of patients experiencing severe vomitingand myelosuppression, and severediarrhea seen in 12%.[20]The popular German ArbeitsgemeinschaftInternische Onkologie(AIO) schedule of high-dose 5-FUadministered as a 24-hour infusion weekly has also been combined withirinotecan. Vanhoefer et al[21] deliveredthe full dose of 5-FU (2,600 mg/m2 weekly) and leucovorin (500 mg/m2) with 80 mg/m2 of irinotecan. Thedose-limiting toxicity was severe diarrhea;however, myelosuppressionwas not a significant problem.[21]Other researchers have evaluatedfurther combinations of the agents.Falcone et al[22] combined a 48-hourcontinuous infusion of 5-FU (3,500mg/m2) with irinotecan in 33 patients,evaluating irinotecan both precedingand following 5-FU. Cycles prior to5-FU and leucovorin permitted a higherdose of irinotecan administration(recommending a dose of 350 mg/m2)in this combination, with less toxicityoverall than the reverse schedule. Severeneutropenia was noted in 22% ofpatients, and grade 3/4 diarrhea in4%.[22] In a phase I study in 42 patientswith metastatic colon cancer,Kakolyris et al[23] combined a 4-daycontinuous infusion of 5-FU withirinotecan immediately afterwards.The doses recommended for subsequentevaluation were 600 mg/m2/dand 350 mg/m2, respectively. At thesedoses, 20% of 25 cycles reported grade3/4 neutropenia, and dose-limitingtoxicities were noted in two of sixpatients: severe neutropenia with severediarrhea, and neutropenic fever.[23]Capecitabine (Xeloda), an oral fluoropyrimidine,was found to have superioractivity and less toxicity incomparison to bolus 5-FU and leucovorinadministered on the Mayo clinicschedule.[24,25] Because of its easeof administration and good toxicityprofile, capecitabine has been combinedwith irinotecan, with promisingresults. Several schedules have beenevaluated. Cassata et al[26] combined1,000 mg/m2 of capecitabine twicedaily for 14 days with irinotecan, withthe latter administered either as 300mg/m2 on day 1 or 150 mg/m2 on days1 and 8 of each 21-day treatment cycle.Both schedules were fairly welltolerated, and active in the first-linetreatment setting (71% overall response[15/21]).[26]Others have evaluated similarschedules and slightly lower irinotecandoses yielding similar findingswith regard to efficacy, as well as asuggestion of somewhat better toxicityprofiles.[27,28]. On the formerschedule, Delord et al[29] used 250mg/m2 of irinotecan on day 1 of treatment,and reported grade 3 neutropeniain two of seven patients, and grade3 diarrhea in one patient. As part of arandomized phase II study, Jordan etal[30] treated advanced colorectal cancerpatients with irinotecan at 100 mg/m2 on days 1 and 8 of each 21-daytreatment cycle. Severe diarrhea wasreported in three and severe neutropeniain two of the 28 patients. However,two patients died from neutropenicsepsis with diarrhea and pulmonaryembolism, respectively.[30]Despite evaluations of these variousschedules, the preferred combinationof irinotecan and 5-FU remainsuncertain. The schedules that havebeen the most intensely evaluated todate are the weekly IFL schedule, andirinotecan in combination with somevariation of the bimonthly de Gramontschedule.IFL as First-Line Therapyin Colorectal CancerEfficacy
The superior antitumor activity ofIFL in comparison to 5-FU and leucovorinadministered by the Mayo or deGramont schedules, as well as irinotecanalone in patients with metastaticcolorectal cancer with no priorchemotherapy for metastatic disease,was established by two reports publishedin 2000. The first by Saltz etal,[18] in the New England Journal ofMedicine as described above, demonstratedsuperior response, time to progression,and survival with theaddition of irinotecan to 5-FU andleucovorin in comparison to irinotecanalone, or 5-FU and leucovorinadministered according to the Mayoclinic schedule.Similarly, Douillard et al[31] randomized387 patients to one of two 5-FU/leucovorin treatment regimens (deGramont schedule with this schedule[288 patients] or AIO schedule [97patients] at the investigator's discretion)with or without irinotecan. Irinotecanwas administered at either 180mg/m2 IV on day 1 of therapy every 2 weeks with the de Gramont schedule,or 80 mg/m2 IV weekly. Again, irinotecansignificantly increased the responserate (P < .005), time toprogression (P < .001), and mediansurvival (P < .031) in comparison topatients treated with 5-FU and leucovorinalone, regardless of the scheduleemployed.[31]With these studies demonstratingthe efficacy of IFL, this combinationsubsequently became the standard initialtherapy for patients with metastaticcolorectal cancer. Although thepreferred combination was uncertain,in the United States the weekly schedulewas most widely used, in part becauseof the ease of administration.However, subsequent reports haveraised concerns about the tolerabilityof this schedule.Toxicity
Not surprisingly, the toxicity profileof IFL depends in great part onthe schedule of 5-FU administeredwith irinotecan (Table 2). The weeklyIFL toxicities reported by Saltz etal[18] in the phase III study were primarilymyelosuppression, with 53.8%of patients experiencing grade 3/4 neutropenia,and neutropenic fevers in7.1%. Severe or life-threatening diarrheawas also a prominent toxicity,occurring in 22.7% of patients, andgrade 3/4 nausea/vomiting in 9.7%.Overall though, therapy was consideredto be well tolerated, with only 2(0.9%) of 225 patients dying as a consequenceof therapy.[18]With the combination of the deGramont schedule of 5-FU and leucovorinwith irinotecan administeredevery other week, grade 3/4 neutropeniaremained the most common toxicity,occurring in 46.2% of patients,with neutropenic fever in 5.5% of patients.Severe diarrhea occurred in13.1% of patients, and grade 3/4 nausea/vomiting in about 3% of patients.When combined with 5-FU and leu-
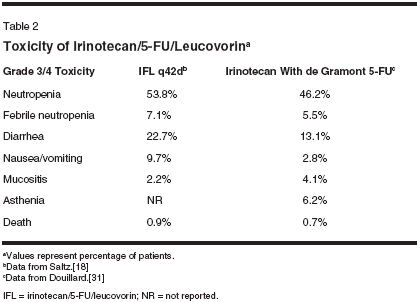
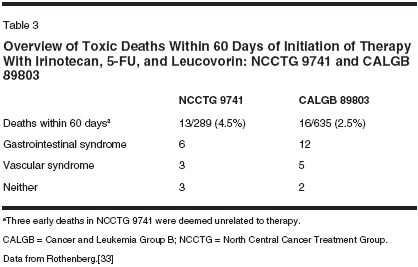
covorin administered on the AIOschedule, IFL resulted in somewhatmore toxicity, including severe diarrheain 44.4% and vomiting in 11.1%of the 54 patients treated. Grade 3/4neutropenia was reported in 28.8%,and febrile neutropenia in 9.3%.[31]This difference in toxicities amongthese regimens was most likely a consequenceof the 5-FU/leucovorinschedule employed, and the resultantdifference in irinotecan schedule.Again, the regimens appeared to havea similar efficacy, despite the differencein toxicity.With the combination of irinotecan,5-FU, and leucovorin becomingthe standard therapy for patients withmetastatic colorectal cancer, its use inthe late 1990s and early 21st centuryescalated dramatically. In the UnitedStates, the weekly regimen of IFL,the so-called Saltz regimen, had becomethe predominant schedule employedbecause of the relative ease ofadministration, which did not requirethe placement of prolonged venousaccess. However, dramatic reportsfrom two Intergroup studies-Cancerand Leukemia Group B (CALGB)89803 and North Central CancerTreatment Group (NCCTG) 9741-evaluating the efficacy of this scheduleof IFL in the respective adjuvantand metastatic settings have led torenewed concerns about the tolerabilityof this regimen (Table 3).In April 2001, the External DataMonitoring Committee for NCCTG9741 reported deaths within the first60 days of study entry in 13 (4.5%) of289 patients. A subsequent review ofthe CALGB study found that 16(2.5%) of 635 patients treated withIFL also died within 60 days of initiatingtreatment. Of interest, the initialreport of IFL in a phase III studynoted that 0.9% of 225 patients diedfrom drug-related causes, comparedto 1.4% of 219 patients treated withbolus 5-FU/leucovorin on the MayoClinic schedule. The deaths that occurredon this study were later reviewed,and the 60-day mortality, thesame yardstick applied to the Intergroupstudies, revealed rates of 6.7%with IFL and 7.3% with 5-FU/leucovorin.[32]An independent review of thesedeaths attributed them to "gastrointestinalsyndrome," including diarrhea,nausea, vomiting, abdominal crampingleading to dehydration and electrolyteabnormalities, and often in thesetting of neutropenia, fever, or infection;or "vascular syndrome," includingmyocardial infarction, pulmonaryembolism, or cerebrovascular accidents.The gastrointestinal syndromewas felt to cause, exacerbate, or contributeto the deaths of 12 patientsin the CALGB study and 6 in theNCCTG study. The vascular syndromewas believed to cause or contributeto the deaths of five patients inthe CALGB study and three in theNCCTG study.The panel found that the medianage of the patients treated with IFLwho died was 69.5 years in CALGB89803 and 65 years in NCCTG 9741,older than the median ages of patientstypically enrolled in studies of colorectalcancer. A number of recommendationswere made by this expertpanel, including close monitoring ofpatients treated with IFL, especiallyolder patients, and an aggressive approachto the treatment of diarrheaand abdominal cramping, includingaggressive use of antibiotics in patients with diarrhea and neutropenia.[33]In addition to the concerns aboutthe toxicities of weekly IFL, anotherdifficulty of the regimen is that severetoxicities occurred despite a relativelylow dose intensity ofchemotherapy. In particular, great difficultywas encountered in administeringweeks 3 and 4 of chemotherapybecause of myelosuppression and diarrhea.As a result, the median relativedose intensities (calculated bydividing the actual dose of the agentdelivered by the intended dose of theagent) of irinotecan and 5-FU were72% and 71%, respectively.[18]21-Day Schedule
Considering the difficulties of dosedelivery and toxicity in weekly IFLaccording to the Saltz schedule, whichappeared to be cumulative within acycle, one manner of improving thetherapeutic index of weekly IFL wouldseem to be to create a break after thesecond week of therapy, prior to resumingIFL. To evaluate this hypothesis,23 patients have been treated withweekly IFL at the Lombardi CancerCenter at Georgetown UniversityMedical Center. However, therapywas administered on days 1 and 8every 21 days. For patients who were75 years or older, the initial dose ofirinotecan was 100 mg/m2. Theplanned dose intensity of this schedulewould be identical to the Saltzschedule of IFL.The patient population was similarto other studies of patients with ad-
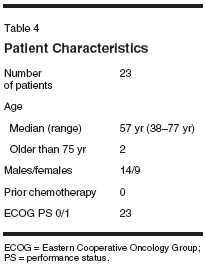
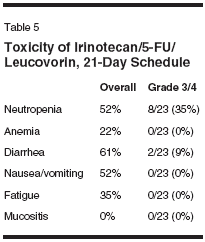
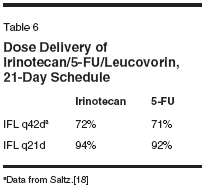
vancedcolorectal cancer (Table 4).However, none of the patients hadreceived prior chemotherapy. All patientshad a good performance status(Eastern Cooperative Oncology Group0 or 1). The median age of the populationwas 57 years, encompassing arange of ages from 38 to 77; two patientswere 75 years or older. Fourteenof the patients were males. Fifteenof the patients were given chemotherapyas adjuvant treatment. Only eightof these patients received therapy astreatment for measurable metastaticdisease. One patient has received 6weeks of therapy and is not yet evaluablefor response. Three of the otherseven had stable disease, and four hadprogression of disease on their follow-up evaluation.This schedule was well tolerated,with grade 3/4 neutropenia occurringin eight (35%) patients, and severediarrhea in only two (9%). Two patientsexperienced one episode eachof febrile neutropenia with the firstcycle of therapy, but tolerated furthertreatment with IFL on the 21-dayschedule after a 25% dose reduction.No other grade 3/4 toxicities werenoted (Table 5).Supporting these data that demonstratethe tolerability of this scheduleof IFL was the ability to deliver thetherapy. In the first 18 patients treatedwith this schedule, the median relativedose intensities, calculated by thesame method as Saltz et al,[18] ofirinotecan and 5-FU were 94% and 92% (Table 6). Half of these patientsreceived therapy without requiring anydose modifications. Full doses wereadministered in 104 of 141 cycles,with a 10% dose reduction occurringin 26 cycles (18.4%), and 25% and50% dose reductions in 9 and 2 cycles,respectively.These results, especially with regardto the ability to deliver a highdose intensity of the regimen with asimple modification of the scheduleof administration, support the hypothesisthat altering the schedule of therapywill improve the therapeutic indexof IFL. However, because of the potentiallyconfounding differences betweenstudy populations, thecomparison of median relative doseintensity between these two studygroups requires confirmation in a prospectiverandomized study. Furthermore,the change in the schedule maynot be the only, or primary, reason forthe ability to deliver such a high proportionof the intended dose.In particular, the patient populationmust be considered to be favorable.First, the median age of thetreated patients was 57 years, withonly two patients being over 75, andthus may be considered inadequatelyrepresentative of the population ofpatients with advanced colorectal cancer.Moreover, as many of the patientswho were treated in this programhad only a high risk for disease recurrence,and essentially received adjuvanttherapy, they may have been a"healthier" population overall. Theantitumor activity and tolerability ofthe 21-day schedule of IFL, then, canonly be assessed in the context of aprospective randomized trial.
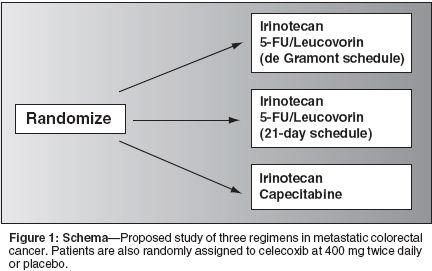
Future DirectionsAt the 2002 meeting of the AmericanSociety of Clinical Oncology,Goldberg et al[34] reported the preliminaryresults of NCCTG 9741. Atotal of 795 patients with advancedcolorectal cancer were randomized toweekly IFL using the Saltz scheduleas the control arm, or oxaliplatin(Eloxatin), 5-FU, and leucovorin onthe de Gramont schedule (FOLFOX4), or a combination of irinotecan andoxaliplatin every 3 weeks. The medianprogression-free survival and medianoverall survival weresignificantly longer for patients treatedwith FOLFOX 4 than IFL on theSaltz schedule, at 8.8 vs 6.9 months,and 18.6 compared to 14.1 months,respectively.[34]As a result of these findings, theUS Food and Drug Administrationapproved oxaliplatin in August 2002for use in combination with infusional5-FU and leucovorin for the treatmentof patients with advancedcolorectal cancer. An additional questionis whether oxaliplatin-based chemotherapywill become the newstandard first-line therapy for patientswith metastatic colorectal cancer.Irinotecan, oxaliplatin, and 5-FU possessactivity in advanced colorectalcancer, and should be made availableto all. However, the appropriate combinationand best sequence of theseagents will need to be elucidated, including the optimal method of administering5-FU (ie, bolus, infusional, ororal) (Figure 1).Finally, the potential role of thetargeted therapies, such as the epidermalgrowth factor receptor (EGFR) antagonistsincluding erbitux (C-225),[35]gefitinib (ZD1839, Iressa), and OSI-774 (Tarceva), and vascular endothelialgrowth factor antagonistsincluding bevacizumab,[36] are beingevaluated. About 70% of patientswith colorectal cancer have tumorsthat overexpress EGFR, making thisa promising target for intervention.Preliminary studies have suggestedthat the combination of irinotecan anderbitux has activity in patients withmetastatic colorectal cancer. The precisecontribution of erbitux in thiscombination, as well as the optimalmethod of combining chemotherapyand these targeted therapies, also remainunknown [35].With a plethora of other potentialtargets and agents against these targetsbeing identified and developed, avariety of options may be availablefor patients in the future, offering anopportunity to tailor therapy to thepatient, maximize activity, and minimizetoxicity. A modification of theweekly bolus IFL, altering the scheduleto administer therapy on days 1and 8 every 21 days, may improve thetherapeutic index of IFL and allowphysicians to continue offering patientsa relatively easily delivered and effective chemotherapy regimen, andencourage investigators to explore theregimen as a backbone to further studyof new agents and combinations inthe treatment of advanced colorectalcancer.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.
Rothenberg ML, Kuhn JG, Burris III HA,et al: Phase I and pharmacokinetic trial of weeklyCPT-11. J Clin Oncol 11(11):2194-2204,1993.
2.
Rougier P, Bugat R, Douillard JY, et al:Phase II study of irinotecan in the treatment ofadvanced colorectal cancer in chemotherapynaïvepatients and patients pretreated with fluorouracil-based chemotherapy. J Clin Oncol15(1):251-260, 1997.
3.
Pitot HC, Wender DB, O’Connell MJ, etal: Phase II trial of irinotecan in patients withmetastatic colorectal carcinoma. J Clin Oncol15(8):2910-2919, 1997.
4.
Kohne CH, Thuss-Patience P, Catane R,et al: Final results of a phase II trial of CPT-11in patients with advanced gastric cancer (abstract993). Proc Am Soc Clin Oncol 18:258a,1999.
5.
Ajani JA, Baker J, Pisters PWT, et al:CPT-11 plus cisplatin in patients with advanced,untreated gastric or gastroesophageal junctioncarcinoma. Results of a phase II study. Cancer94(3):641-646, 2002.
6.
Ilson DH, Saltz L, Enzinger P, et al: PhaseII trial of weekly irinotecan plus cisplatin inadvanced esophageal cancer. J Clin Oncol17(16):3270-3275, 1999.
7.
Wagener DJ, Verdonk HE, Kirix LY, etal: Phase II trial of CPT-11 in patients withadvanced pancreatic cancer, an EORTC earlyclinical trials group study. Ann Oncol 6(2):129-132, 1995.
8.
Baker L, Khan R, Lynch T, et al: Phase IIstudy of irinotecan (CPT-11) in advanced nonsmallcell lung cancer (NSCLC) (abstract 1658).Proc Am Soc Clin Oncol 16:461a, 1997.
9.
DeVore RF, Blanke CD, Denham CA, etal: Phase II study of irinotecan (CPT-11) inpatients with previously treated small cell lungcancer (SCLC) (abstract 1736). Proc Am SocClin Oncol 17:451a, 1998.
10.
Perez EA, Hillman DW, Mailliard JA, etal: Randomized phase II study of 2 schedulesof irinotecan (CPT-11) for patients (pts) withrefractory metastatic breast cancer (MBC): AnNCCTG Cooperative Group study (abstract206). Proc Am Soc Clin Oncol 21:52a, 2002.
11.
Vershraegen CF, Levy T, Kudelka AP,et al: Phase II study of irinotecan in prior chemotherapy-treated squamous cell carcinoma ofthe cervix. J Clin Oncol 15(2):625-631, 1997.
12.
Bodurka-Bevers D, Levenback C, Wolf J,et al: A phase II trial of irinotecan (CPT-11) inpatients with metastatic epithelial ovarian cancer(EOC) or peritoneal cancer (PC) (abstract864). Proc Am Soc Clin Oncol 20:217a, 2001.
13.
Cunningham D, Pyrhonen S, James RD,et al: Randomised trial of irinotecan plus supportivecare versus supportive care alone after fluorouracil failure for patients with metastaticcolorectal cancer. Lancet 352(9138):1413-1418,1998.
14.
Rougier P, Van Cutsem E, Bajetta E, etal: Randomised trial of irinotecan versus fluorouracilby continuous infusion after fluorouracilfailure with metastatic colorectal cancer.Lancet 352(9138):1407-1410, 1998.
15.
Conti JA, Kemeny NE, Saltz LB, et al:Irinotecan is an active agent in untreated patientswith metastatic colorectal cancer. J ClinOncol 14(93):709-715, 1996.
16.
Firvida JL, Irigoyen A, Vazquez-EstevezS, et al: Phase II study of irinotecan as first-linechemotherapy for patients with advanced colorectalcarcinoma. Cancer 91(4):704-711, 2001.
17.
Saltz LB, Kanowitz J, Kemeny NE, et al:Phase I clinical and pharmacokinetic study ofirinotecan, fluorouracil, and leucovorin in patientswith advanced solid tumors. J Clin Oncol14(11):2959-2967, 1996.
18.
Saltz LB, Cox JV, Blanke C, et al: Irinotecanplus fluorouracil and leucovorin for metastaticcolorectal cancer. N Engl J Med343(13):905-914, 2000.
19.
De Gramont A, Bosset JF, Milan C, etal: Randomized trial comparing monthly lowdoseleucovorin and fluorouracil bolus withbimonthly high-dose leucovorin and fluorouracilbolus plus continuous infusion for advancedcolorectal cancer: A French Intergroupstudy. J Clin Oncol 15(2):808-815, 1997.
20.
Andre T, Louvet C, Maindrault-GoebelF, et al: CPT-11 (irinotecan) addition to bimonthly,high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) forpretreated metastatic colorectal cancer. GERCOR.Eur J Cancer 35(5):1343-1347, 1999.
21.
Vanhoefer U, Harstrick A, Kohne C-H,et al: Phase I study of a weekly schedule ofirinotecan, high-dose leucovorin, and infusionalfluorouracil as first-line chemotherapy in patientswith advanced colorectal cancer. J ClinOncol 17(3): 907-913, 1999.
22.
Falcone A, Di Paolo A, Masi G, et al:Sequence effect of irinotecan and fluorouraciltreatment on pharmacokinetics and toxicity inchemotherapy-naïve metastatic colorectal cancerpatients. J Clin Oncol 19(15):3456-3462,2001.
23.
Kakolyris S, Souglakos J, Kouroussis C,et al: A dose finding study of irinotecan (CPT-11) plus a four-day continuous 5-fluorouracilinfusion in advanced colorectal cancer. Oncology(Basel) 60(3):207-213, 2001.
24.
Hoff PM, Ansari R, Batist G, et al: Comparisonof oral capecitabine versus intravenousfluorouracil plus leucovorin as first-line treatmentin 605 patients with metastatic colorectalcancer: Results of a randomized phase III study.J Clin Oncol 19(8):2282-2292, 2001.
25.
Van Cutsem E, Twelves C, Cassidy J, etal: Oral capecitabine compared with intravenousfluorouracil plus leucovorin in patientswith metastatic colorectal cancer: Results of alarge phase III study. J Clin Oncol 19(21):4097-4106, 2001.
26.
Cassata A, Chiara Stani S, Alu M, et al:Ongoing phase II trial with two schedules ofirinotecan (CPT-11) in combination withcapecitabine as first line chemotherapy of patientswith advanced colorectal cancer (ACRC)(abstract 573). Proc Am Soc Clin Oncol 20:144a,2001.
27.
Schleucher N, Tewes M, Achterrath W,et al: Extended phase I study of capecitabine incombination with a weekly schedule of irinotecanas first-line chemotherapy in metastaticcolorectal cancer (abstract 561). Proc Am SocClin Oncol 20:141a, 2001.
28.
Vanhoefer UJ, Mayer S, Achterrath W,et al: Phase I study of capecitabine in combinationwith a weekly schedule of irinotecan asfirst-line chemotherapy in metastatic colorectalcancer (abstract 212P). Ann Oncol 11(suppl4):49, 2000.
29.
Delord JP, Pierga JY, Dieras V, et al:Dose escalation and pharmacokinetic study ofcapecitabine (Xeloda) and irinotecan (CPT-11)in gastro-intestinal tumors: Preliminary results(abstract 397). Proc Am Soc Clin Oncol 21:100a,2002.
30.
Jordan K, Grothey A, Kellner O, et al:Randomized phase II trial of capecitabine plusirinotecan vs capecitabine plus oxaliplatin asfirst-line therapy in advanced colorectal cancer(ACRC): Results of an interim analysis (abstract2225). Proc Am Soc Clin Oncol 21:103b,2002.
31.
Douillard JY, Cunningham D, Roth AD,et al: Irinotecan combined with fluorouracilcompared with fluorouracil alone as first-linetreatment for metastatic colorectal cancer: Amulticentre randomized trial. Lancet355(9212):1041-1047, 2000.
32.
Miller LL: Recommendation for cautionwith irinotecan, fluorouracil, and leucovorinfor colorectal cancer. N Engl J Med 345(2):146,2001.
33.
Rothenberg ML, Meropol NJ, PoplinEA, et al: Mortality associated with irinotecanplus bolus fluorouracil/leucovorin: Summaryfindings of an independent panel. J Clin Oncol19(18):3801-3807, 2001.
34.
Goldberg RM, Morton RF, Sargent DJ,et al: N9741: Oxaliplatin (oxal) or CPT-11 + 5-fluorouracil (5-FU)/leucovorin (LV) or oxal +CPT-11 in advanced colorectal cancer (CRC).Initial toxicity and response data from a GIIntergroup study (abstract 511). Proc Am SocClin Oncol 21:128a, 2002.
35.
Saltz LB, Rubin M, Hochster H, et al:Cetuximab (IMC-C225) plus irinotecan (CPT-11) is active in CPT-11 refractory colorectalcancer (CRC) that expresses epidermal growthfactor receptor (EGFR) (abstract 7). Proc AmSoc Clin Oncol 20:3a, 2001.
36.
Bergsland E, Hurwitz H, FehrenbacherL, et al: A randomized phase III trial comparingrhuMAb VEGF (recombinant humanizedmonoclonal antibody to vascular endothelialcell growth factor) plus 5-fluorouracil/leucovorin(FU/LV) to FU/LV alone in patients withmetastatic colorectal cancer (abstract 939).Proc Am Soc Clin Oncol 19:242a, 2000.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.