Gemcitabine and Irinotecan in Locally Advanced or Metastatic Biliary Cancer: Preliminary Report
Chemotherapy has had limited success in biliary tract cancer. Of thenewer agents, gemcitabine (Gemzar) and irinotecan (CPT-11, Camptosar)both have single-agent activity in patients with advanced disease.We conducted a phase II trial to study the efficacy and toxicity of thecombination of gemcitabine plus irinotecan in patients with locallyadvanced or metastatic biliary tract cancer. The study has enrolled 14patients with histologically or cytologically documented cancer of thebiliary tract or gallbladder with bidimensionally measurable disease,Eastern Cooperative Oncology Group performance status 0 or 1,decompressed biliary tree, and no prior exposure to chemotherapy.Gemcitabine at 1,000 mg/m2 and irinotecan at 100 mg/m2 were bothadministered on days 1 and 8, every 21 days. In patients who had lessthan grade 3 hematologic and less than grade 2 nonhematologic toxicityfollowing cycle 1, the dose of irinotecan was increased to 115 mg/m2 forsubsequent cycles. A total of 65 cycles of chemotherapy have beenadministered, with an average of 4.5 cycles per patient (range: 1 to 11cycles). The median treatment duration was 3 months (range: 0.75 to 8months). An objective partial response was determined radiographicallyin two patients (14%) while stable disease for periods ranging from 4to 11.5 months was noted in six patients (43%). Toxicity consisted ofgrade 3/4 neutropenia in seven patients (50%) with no episodes offebrile neutropenia, grade 3/4 thrombocytopenia in four (28%), grade3 diarrhea in two (14%), and grade 3 nausea in one patient. Thecombination of gemcitabine plus irinotecan appears to possess modestclinical activity, and it is well tolerated in patients with advanced biliarycancer. Patient accrual is ongoing to this study.
ABSTRACT: Chemotherapy has had limited success in biliary tract cancer. Of thenewer agents, gemcitabine (Gemzar) and irinotecan (CPT-11, Camptosar)both have single-agent activity in patients with advanced disease.We conducted a phase II trial to study the efficacy and toxicity of thecombination of gemcitabine plus irinotecan in patients with locallyadvanced or metastatic biliary tract cancer. The study has enrolled 14patients with histologically or cytologically documented cancer of thebiliary tract or gallbladder with bidimensionally measurable disease,Eastern Cooperative Oncology Group performance status 0 or 1,decompressed biliary tree, and no prior exposure to chemotherapy.Gemcitabine at 1,000 mg/m2 and irinotecan at 100 mg/m2 were bothadministered on days 1 and 8, every 21 days. In patients who had lessthan grade 3 hematologic and less than grade 2 nonhematologic toxicityfollowing cycle 1, the dose of irinotecan was increased to 115 mg/m2 forsubsequent cycles. A total of 65 cycles of chemotherapy have beenadministered, with an average of 4.5 cycles per patient (range: 1 to 11cycles). The median treatment duration was 3 months (range: 0.75 to 8months). An objective partial response was determined radiographicallyin two patients (14%) while stable disease for periods ranging from 4to 11.5 months was noted in six patients (43%). Toxicity consisted ofgrade 3/4 neutropenia in seven patients (50%) with no episodes offebrile neutropenia, grade 3/4 thrombocytopenia in four (28%), grade3 diarrhea in two (14%), and grade 3 nausea in one patient. Thecombination of gemcitabine plus irinotecan appears to possess modestclinical activity, and it is well tolerated in patients with advanced biliarycancer. Patient accrual is ongoing to this study.
Biliary tract cancers include adenocarcinomasof the gallbladderand bile ducts (cholangiocarcinoma,both intra- and extrahepatic).Cancers of the biliary treeare relatively uncommon in NorthAmerica, with approximately 8,000new cases diagnosed in 2003.[1]While surgical resection is potentiallycurative, only about 25% of patientsare resectable at the time ofpresentation. Furthermore, there is ahigh rate of both local and systemicrelapse after a potentially curative resection.Unresectable or metastaticbiliary tract carcinoma carries a poorprognosis due to the lack of effectivetherapy. Patients with advanced gallbladdercancer have a median survivalof approximately 6 months, whilethose with cholangiocarcinoma mayhave a more indolent course with amedian survival duration of about 1year.[2]Several chemotherapy regimenshave been explored in patients withadvanced biliary cancers. However,the published series are small, andthey often include a heterogeneouspopulation of patients with both pancreaticand hepatocellular cancers aswell as those with biliary cancers. Useof fluorouracil (5-FU)-based regimensresult in a response rates ranging from10% to 30% but with limited responseduration and short median survival (9to 12 months). In a study using continuous infusion of 5-FU and recombinanthuman interferon alfa-2b(n = 32), Patt et al reported an objectiveresponse rate of 34% with mediansurvival of 12 months.[3] However,there was substantial toxicity with thisregimen. In a subsequent study by thesame group (n = 41), the addition ofcisplatin and doxorubicin to 5-FU andrecombinant human interferon alfa-2b resulted in a lower overall responserate (21%) with median survival of14 months.[4]
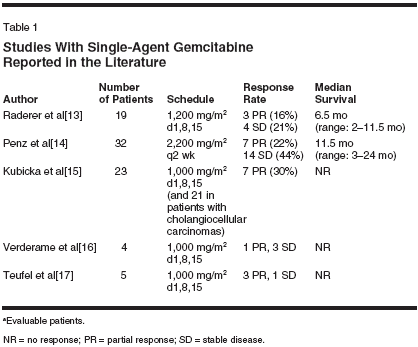
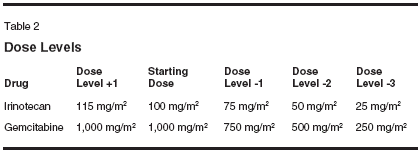
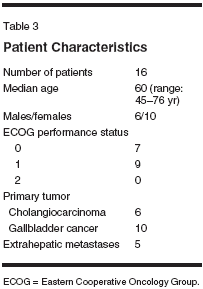
Both gemcitabine (Gemzar) andirinotecan (CPT-11, Camptosar) possesssingle-agent activity in biliarytract cancers. Although the availablestudies are small (Table 1), responserates with single-agent gemcitabinehave consistently been in the range of16% to 36% with median survivalranging from 6 to 11 months. Severalpatients treated with gemcitabine experiencedprolonged periods of diseasestabilization. Two studies usingsingle-agent irinotecan have been reportedin the literature. In one study(n = 25) using irinotecan at 125 mg/m2 once a week for 4 consecutiveweeks followed by a 2-week break,Sanz-Altamira et al reported an 8%partial response rate and 40% stabledisease lasting at least 2 months.[5]An ongoing study by the North CentralCancer Treatment Group (n = 13)showed substantial toxicity withirinotecan in patients with biliary cancers,while response rates were notreported.[6]Preclinical studies show a dosedependentinteraction between gemcitabineand irinotecan, withsynergism at clinically relevant concentrations.[7,8] Based on the singleagentactivity of both agents in biliarycancers, preclinical evidence of synergy,and the lack of overlapping toxicity,we tested the combination ofgemcitabine and irinotecan in patientswith advanced biliary tract cancers.The dose and schedule used in thisstudy were based on a prior phase Istudy conducted by Rocha Lima et al.[9]Patients And MethodsPatients with previously untreated,unresectable histologically or cytologicallydocumented cancer of thebiliary tract (intrahepatic or extrahepatic)or gallbladder were eligible forparticipation. Patients were requiredto have measurable or evaluable diseaseand Eastern Cooperative OncologyGroup (ECOG) performancestatus of 0 to 2. Adequate bone marrowfunction (granulocyte > 1,500/μL,platelets > 100,000/μL), hepatic function(serum bilirubin < 2.0 mg/dL),and renal function (serum creatinine< 2.0 mg/dL) were also required. Patientswith an active second malignancy (other than nonmelanoma skincancer) or serious medical or psychiatricdisease were excluded. The studywas approved by the local institutionalreview board at each participatinginstitution, and informed consent wasobtained from all patients.Treatment PlanGemcitabine at 1,000 mg/m2 wasgiven intravenously (IV) over 30minutes followed by irinotecan at100 mg/m2 IV over 90 minutes ondays 1 and 8 every 21 days. In theabsence of grade 2 or higher nonhematologictoxicity (except for nausea/vomiting, alopecia, anorexia, andfever), or grade 3 or higher hematologictoxicity during cycle 1, the doseof irinotecan was escalated to 115 mg/m2 for subsequent cycles. Standardpremedication included dexamethasoneand a 5-HT3 receptor antagonist,while atropine was not given routinelyunless patients experienced an episodeof early diarrhea. All patientswere instructed to take loperamide atthe earliest signs of diarrhea and/orabdominal cramping that occurredmore than 24 hours after receivingirinotecan. Dose adjustments weremade independently for gemcitabineand irinotecan depending on the typesof toxicity observed (Table 2). Tumormeasurements were performed everytwo cycles, and standard response criteriawere used.ResultsSixteen patients have been enrolledin this study (10 females and 6 males).Six patients had cholangiocarcinoma,and 10 patients had gall bladder cancer.The median age was 60 years(range: 45-76 years) and all patientshad an ECOG performance status of 0or 1. Extrahepatic metastases werepresent in five patients (Table 3).A total of 65 cycles of chemotherapyhave been administered, with anaverage of 4.5 cycles per patient(range: 1-11 cycles). The mediantreatment duration of treatment was3 months (range: 0.75-8 months). Anobjective partial response was determinedradiographically in two patients(14%), while stable disease for periods ranging from 4 to 11.5 monthswas noted in six patients (43%). Themedian time to progression was 1.5months (range: 1-11.5 mo). Patientaccrual is ongoing, with a target of 25patients.The major toxicity was gastrointestinaland myelosuppression. Two patients(14%) had grade 3 diarrhea andone patient had grade 3 nausea. Grade3/4 neutropenia occurred in 7 patients(50%) with no episodes of febrile neutropenia,and grade 3/4 thrombocytopeniaoccurred in four patients(28%). There were 2 deaths duringtreatment one attributed to bowel obstructionsecondary to disease progression,and one due to pneumoniawithout neutropenia.Discussion and ConclusionThe combination of gemcitabineand irinotecan represents a new optionin the treatment of biliary tractcancers. The activity of gemcitabinein biliary tract cancers was suggestedin case reports several years ago. Castroreported a single patient with hepaticand intraperitoneal metastasesfrom gall bladder cancer who hadfailed two other chemotherapeutic regimens.He had dramatic response tosingle-agent gemcitabine with completereversal of small bowel obstructionand the disappearance of hepaticmetastases.[10]In one of the earliest trials, Mezgeret al treated 13 patients with gallbladderand biliary tract with gemcitabineat 1,000 mg/m2 weekly for 7 weeks,then weekly for 3 out of 4 weeks untildisease progression.[11] Althoughonly one patient had a partial response,11 (85%) patients had stable diseasewith a median time to progression of7 months and median overall survivalof 11 months. Several subsequentstudies and case reports have demonstratedconsistent activity of gemcitabinein biliary tract cancers.[12]Single-agent irinotecan has modestclinical activity in biliary tractcancers.[5]Preclinical studies evaluating combinationsof anticancer drugs report adose-dependent interaction betweengemcitabine and irinotecan.[8] In vitrostudies show antagonism at low concentrations (< 0.1 μM) , but synergismat concentrations of gemcitabineabove 0.1 μM and irinotecan above3.2μM in the SCOG small-cell lungcancer cell line.[7] Absolute, markedsynergism was evident in the HL-60acute myeloid leukemia cell line.Synergism at concentrations of 0.1 to2 μM gemcitabine and 0.2 to 10 μMirinotecan, but antagonism at high concentrations(ie, concentrations > 2 μMgemcitabine and 20 μM irinotecan),was seen in MCF7 breast cancercells.[7]Phase I studies evaluating the combinationof gemcitabine and irinotecanon a day 1 and 8 schedule reporteda maximum tolerated dose of 1,000mg/m2 gemcitabine and 100 mg/m2irinotecan.[9] Escalation of irinotecanto 115 mg/m2 was recommended forsubsequent cycles in patients withminimal or no toxicity during the firstcycle. The sequence of administrationof gemcitabine followed by irinotecanwas empirical. No preclinicaldata were available to suggest sequence-related differences in toxicityor efficacy. This combination was subsequentlytested in pancreatic and othercancers and was demonstrated tobe clinically active with manageabletoxicity. While a previous phase IIstudy showed improvement in responserate with the combination ofgemcitabine and irinotecan in pancreaticcancer,[18] a subsequent phaseIII randomized study failed to showany improvement in survival with thiscombination compared with singleagentgemcitabine.[19]In summary, our phase II trial suggeststhat the combination of gemcitabineand irinotecan has modestclinical activity and is well toleratedin patients with advanced or metastaticbiliary cancer. Patient accrual isongoing to this study.
Disclosures:
The author(s) have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1.
Jemal A, Murray T, Samuels A, et al:Cancer statistics, 2002. CA Cancer J Clin 53:5-26, 2003.
2.
Pitt HA, Grochow LB, Abrama RA: Cancerof the biliary tree, in DeVita VT, HellmanS, Rosenberg SA (eds): Cancer, Principles andPractice of Oncology, 5th ed, pp 1114-1128.Philadelphia, Lippincott-Raven, 1997.
3.
Patt YZ, Jones DV, Jr, Hoque A, et al: Phase II trial of intravenous fluorouracil andsubcutaneous interferon alfa-2b for biliary tractcancer. J Clin Oncol 14:2311-2315, 1996.
4.
Patt YZ, Hassan MM, Lozano RD, et al:Phase II trial of cisplatin, interferon alpha-2b,doxorubicin, and 5-fluorouracil for biliary tractcancer. Clin Cancer Res 7: 3375-3380, 2001.
5.
Sanz-Altamira PM, O’Reilly E, StuartKE, et al: A phase II trial of irinotecan (CPT-11) for unresectable biliary tree carcinoma. AnnOncol 12:501-504, 2001.
6.
Alberts SR, Mahoney MR, Fishkin PA, etal: Toxicity of irinotecan (CPT-11) in patients(pts) with advanced gallbladder (GB) or biliary(BILI) tumors: A North Central Cancer TreatmentGroup (NCCTG) study (abstract 1166).Proc Am Soc Clin Oncol 19:298a, 2000.
7.
Bahadori HR, Rocha Lima CM, GreenMR, et al: Synergistic effect of gemcitabineand irinotecan (CPT-11) on breast and smallcell lung cancer cell lines. Anticancer Res19:5423-5428, 1999.
8.
Kanzawa F, Saijo N: In vitro interactionbetween gemcitabine and other anticancer drugsusing a novel three-dimensional model. SeminOncol 24(2 suppl 7):S7/8-S7/16, 1997.
9.
Rocha Lima CM, Leong SS, ShermanCA, et al: Irinotecan and gemcitabine in patientswith solid tumors: Phase I trial. Oncology16(5 suppl 5):19-24, 2002.
10.
Castro MP: Efficacy of gemcitabine inthe treatment of patients with gallbladder carcinoma:A case report. Cancer 82:639-641, 1998.
11.
Mezger J, Sauerbruch T, Ko Y, et al:Phase II study with gemcitabine in gallbladderand biliary tract carcinomas Onkologie 21:232-234, 1998.
12.
Scheithauer W: Review of gemcitabinein biliary tract carcinoma. Semin Oncol 29(6suppl 20):40-45, 2002.
13.
Raderer M, Hejna MH, Valencak JB, etal: Two consecutive phase II studies of 5-fluorouracil/leucovorin/mitomycin C and of gemcitabinein patients with advanced biliary cancer.Oncology 56:177-180, 1999.
14.
Penz M, Kornek GV, Raderer M, et al:Phase II trial of two-weekly gemcitabine inpatients with advanced biliary tract cancer. AnnOncol 12:183-186, 2001.
15.
Kubicka S, Rudolph KL, Tietze MK, etal: Phase II study of systemic gemcitabine chemotherapyfor advanced unresectable hepatobiliarycarcinomas. Hepatogastroenterology48:783-789, 2001.
16.
Verderame F, Mandina P, Abruzzo F, etal: Biliary tract cancer: Our experience withgemcitabine treatment. Anticancer Drugs11:707-708, 2000.
17.
Teufel A, Lehnert T, Stremmel W, et al:Chemotherapy with gemcitabine in patients withadvanced gallbladder carcinoma. Z Gastroenterol38:909-912, 2000.
18.
Rocha Lima CM, Savarese D, BrucknerH, et al: Irinotecan plus gemcitabine inducesboth radiographic and CA 19-9 tumor markerresponses in patients with previously untreatedadvanced pancreatic cancer. J Clin Oncol20(5):1182-1191, 2002.
19.
Rocha Lima CMS, Rotche R, Jeffery M,et al: A randomized phase 3 study comparingefficacy and safety of gemcitabine (GEM) andirinotecan (I) to GEM alone in patients withlocally advanced or metastatic pancreatic cancerwho have not received prior systemic therapy(abstract 1005). Proc Am Soc Clin Oncol22:251, 2003.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.