Celecoxib and Radiation Therapy in Non–Small-Cell Lung Cancer
Overexpression of cyclooxygenase-2 (COX-2) is frequently presentin lung cancer and may play a significant role in carcinogenesis, invasion,and metastasis. It has been associated with shortened survival inpatients with resected early-stage adenocarcinoma of the lung. COX-2inhibition decreases tumor cell proliferation in vivo and has been shownto enhance tumor radiosensitivity. Additionally, COX-2 inhibition mayprotect normal pulmonary tissue from radiation fibrosis. Clinical studiesare under way to assess the potential benefits and risks of COX-2inhibition in the treatment of lung cancer. The rationale for COX-2inhibitors in the treatment of lung cancer will be reviewed. The resultsof a phase II study assessing the acute toxicity of concurrent celecoxib(Celebrex) and thoracic irradiation in patients with non–small-cell lungcancer (NSCLC) are reported, and an ongoing Radiation TherapyOncology Group study using celecoxib and concurrent radiation therapyfor NSCLC in patients with intermediate prognostic factors is reviewed.
Overexpression of cyclooxygenase-2 (COX-2) is frequently present in lung cancer and may play a significant role in carcinogenesis, invasion, and metastasis. It has been associated with shortened survival in patients with resected early-stage adenocarcinoma of the lung. COX-2 inhibition decreases tumor cell proliferation in vivo and has been shown to enhance tumor radiosensitivity. Additionally, COX-2 inhibition may protect normal pulmonary tissue from radiation fibrosis. Clinical studies are under way to assess the potential benefits and risks of COX-2 inhibition in the treatment of lung cancer. The rationale for COX-2 inhibitors in the treatment of lung cancer will be reviewed. The results of a phase II study assessing the acute toxicity of concurrent celecoxib (Celebrex) and thoracic irradiation in patients with non–small-cell lung cancer (NSCLC) are reported, and an ongoing Radiation Therapy Oncology Group study using celecoxib and concurrent radiation therapy for NSCLC in patients with intermediate prognostic factors is reviewed.
Cyclooxygenase-2 (COX-2) converts arachidonic acid to prostaglandins. Contrary to COX-1, which is ubiquitous and regulates normal physiologic function, COX-2 is induced by inflammatory stimuli, growth factors, mitogenic substances, and oncogenes.[1] In addition to its role in the inflammatory response, it may play an important role in carcinogenesis.[2,3] Potential mechanisms of carcinogenesis include dysregulation of cell growth, inhibition of apoptosis, interference with immune surveillance, and angiogenesis stimulation.[4,5] COX-2 may also be associated with tumor invasion and metastases[6,7] and has been associated with poor prognosis.[8,9] COX-2 Expression in Lung Cancer Up to 90% of non-small-cell lung cancers (NSCLC) have been shown to express COX-2 at a moderate to strong level.[6,10,11] Although Hida et al reported only a 14% incidence of COX-2 overexpression in squamous cell carcinoma, other investigators report much higher expression.[6] Soslow et al reported COX-2 expression in 11 of 11 squamous cell carcinomas evaluated.[10] The expression level in NSCLC has been shown to be significantly higher than in normal lung tissue for both adenocarcinoma and squamous cell carcinoma.[11] In stage I NSCLC, increased expression of COX-2 has been shown to correlate with shortened survival.[9] COX-2 Inhibitors COX-2 inhibitors have been shown to reduce tumor growth in vitro and in xenografts of human tumor cells.[3,12- 14] Mechanisms include inhibition of tumor cell proliferation, induction of apoptosis, inhibition of neoangiogenesis, and stimulation of antitumor immune response.[5,12,14-16] COX-2 Inhibitors and Radiation TherapyRadiosensitization
Preliminary published results suggest that irradiation upregulates vascular endothelial growth factor (VEGF) and COX-2 production in tumor cells, which in turn stimulates tumor angiogenesis. Additionally, COX-2 upregulated by ionizing radiation can be blocked by the use of a COX-2-specific inhibitor prior to ra diation.[17] Studies have shown that COX-2 inhibition on tumors improved the response to radiotherapy in animal models, possibly through an antiangiogenic mechanism.[17,18] More importantly, this enhancement came without markedly affecting normal tissue radioresponse. The mechanism of COX-2-inhibited radiosensitivity is not completely known. Possible mechanisms of increased radioresponse include inhibition of tumor angiogenesis and inhibition of immunosuppressive activities of prostaglandins.[5] Radioprotective Effect
COX-2 inhibitors do not appear to sensitize normal tissue to radiation and may have a protective effect.[18,19] Pulmonary radiation fibrosis is due in part to inflammatory response to radiation.[ 20] Intuitively, selective inhibition of inflammatory response by COX-2 inhibitors should be protective. Indomethacin, a nonselective COX inhibitor, has been shown to be radioprotective to the lung and hematopoietic system.[21] Clinical Applications in Lung Cancer Combination chemotherapy and radiation for unresectable locally advanced (LA)-NSCLC in patients with favorable prognostic factors is superior to radiation alone.[22-25] Cure rates remain low despite this aggressive therapy. Accordingly, current clinical trials involve increasingly aggressive therapy directed at patients with only the best prognostic factors. Unfortunately, the majority of patients presenting with LA-NSCLC do not meet the stringent eligibility criteria for these studies. Established favorable prognostic factors include Karnofsky performance status ≥ 70 and minimal weight loss. Patient preference, age, physician bias, and subjective evaluation of comorbid conditions also influence choice of therapy. This results in a significant diversity of patient populations categorized by weight loss and Karnofsky status alone. Few studies that evaluate therapies for patients with less favorable prognostic factors have been successfully completed. There are several possible explanations. One is the diversity of the population, which makes study design difficult. Another is physician bias for or against chemotherapy. A phase II Southwest Oncology Group (SWOG) trial treated poor-risk patients with concurrent chemotherapy and radiation with favorable response rates and short-term survival, but with high acute esophageal toxicity.[ 26] Radiation Therapy Oncology Group (RTOG) 97-01 was designed to test the SWOG regimen against standard radiation alone. Accrual was poor due to the reluctance of some physicians to treat patients with radiation alone and that of others to treat with chemotherapy. COX-2 inhibitors are a potential alternative therapy to concurrent chemotherapy in this population. Preclinical data suggest inhibition of cell growth and radiosensitization by COX-2 inhibitors at doses that have minimal systemic toxicity relative to standard chemotherapy agents. An RTOG study is currently open, testing concurrent celecoxib (Celebrex) with limited-field irradiation in patients with LA-NSCLC with an intermediate prognosis. There is no proven benefit to multimodality therapy in these patients. Patients with very poor performance status (Zubrod ≥3) are excluded. Eligible patients include those who do not meet the eligibility criteria for studies intended for patients with good prognostic factors or who refuse chemotherapy. This study also provides an opportunity to prospectively evaluate prognostic factors in order to improve our understanding of this population. Although COX-2 inhibitors are commonly used to treat arthritic conditions with no adverse side effects reported with concurrent radiation, the toxicity of concurrent radiotherapy and celecoxib has not been formally tested. We conducted a feasibility study of concurrent celecoxib and thoracic irradiation at the Medical College of Wisconsin to determine if celecoxib given concurrently with thoracic irradiation increases the acute toxicity expected with radiation therapy alone. Toxicity Evaluation of Concurrent Celecoxib and Thoracic Radiation A phase II study was completed to determine if concurrent celecoxib at 400 mg twice daily with thoracic radiation increases the acute toxicity anticipated with thoracic radiation therapy alone. Materials and Methods
Patients entered in this study were required to have unresectable or medically inoperable NSCLC. Additionally, patients had to have a Karnofsky performance status < 70 and/or > 5% weight loss 3 months prior to diagnosis or they had to be considered inappropriate candidates for concurrent chemotherapy by the treating radiation and medical oncologists. Patients received 400 mg of celecoxib twice daily, 7 days a week, beginning on day 1 of radiation and ending on the last day of radiation. Radiation was delivered once daily, 5 days a week, at 2 Gy per fraction to a total dose of 66 Gy. Radiation fields included the primary tumor and involved regional lymphatics. Uninvolved regional lymphatics were not intentionally included. Patients were followed for acute toxicity for 90 days from the start of therapy, with the exception of one patient who died on day 66. Results
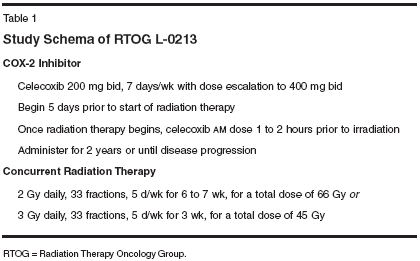
Ten patients completed therapy. Reasons for eligibility included low Karnofsky performance status (3 patients), extensive weight loss (3), poor tolerance to neoadjuvant chemotherapy (1), progressive disease with neoadjuvant chemotherapy (1), and comorbid conditions predicting poor tolerance to concurrent chemotherapy and radiation therapy (3). Three patients had stage IIIa disease, five patients had stage IIIb disease, one patient had stage IV disease (T3, N2 with axillary lymph node metastasis), and one patient had medically inoperable stage Ib disease. None of the patients had progressive locoregional disease during therapy. One patient had progressive disease at 6 months. Two patients had a complete response; one is alive at 11 months with no evidence of disease, and the other died from an unrelated comorbid condition on day 66. Three patients had a partial response to therapy; one is alive without progression at 8 months, and two died from distant metastases without locoregional progression at 2 and 7 months. One patient is alive at 3 months without assessment of disease status. The other three patients died with distant metastases and stable intrathoracic disease. The acute toxicity of celecoxib at 400 mg twice daily with thoracic radiation was not greater than the acute toxicity anticipated with radiation therapy alone. Acute toxicity was restricted to the skin and the esophagus. There was no acute pulmonary toxicity. Skin reactions developed in six patients (grade 1), two patients (grade 2), and two patients (grade 3). Grade 3 skin reactions were at least partially due to the posterior bolus effect of the Alpha Cradle immobilization device. Esophagitis developed in five patients (grade 1), one patient (grade 2), and one patient (grade 3). Grade 3 esophagitis developed in a patient with ex- tensive mediastinal lymphadenopathy, requiring treatment to a long segment of the esophagus. RTOG L-0213 RTOG L-0213 is a phase I/II trial of celecoxib (NSC 719627) with limited field radiation for intermediateprognosis patients with LA-NSCLC, with analysis of prognostic factors. It is open to patients for whom there is no proven benefit to multimodality therapy. Patients with very poor performance status (Zubrod ≥ 3) are excluded. Eligible patients include those who do not meet the eligibility criteria for studies intended for patients with good prognostic factors or who refuse chemotherapy. Patients are treated with concurrent, limitedvolume irradiation and celecoxib followed by 2 years of maintenance therapy. The radiation dose will be either 45 Gy at 3 Gy per fraction or 66 Gy at 2 Gy per fraction at the discretion of the treating physician. The study schema can be found in Table 1.
Study Objectives The objectives of the study are as follows: (1) to determine the toxicity and efficacy of concurrent celecoxib and thoracic irradiation for LANSCLC in intermediate-prognosis patients; (2) to determine how predictors of mortality in the general popu- lation (comorbid conditions, functional status, quality of life, and psychological status) influence prognosis, toxicity of therapy, and outcomes of therapy in patients with LA-NSCLC (this information will be used to develop prognostic indices); (3) to determine if circulating levels of VEGF, basic fibroblast growth factor (bFGF), and interleukin-8 (IL-8) correlate with survival; and (4) to determine if circulating levels of IL-1, IL-6, and transforming growth factor beta (TGF-beta) correlate with the development of pulmonary toxicity. Study Design
The diversity of this patient population makes study design challenging. A more in-depth understanding of prognostic factors will facilitate future study design and individualization of patient care. Factors other than weight loss and performance status will affect prognosis. Comorbid conditions, psychological status, functional status, and quality of life will be evaluated in this study. All of these factors have been shown to have some value in predicting prognosis in oncology and/or nononcology patients.[ 27-31] Additionally, these factors are most likely part of the treating physician's subjective evaluation of patients when making therapeutic decisions, emphasizing the need for prospective evaluation of objective measures. Translational studies in this protocol will seek to determine if (1) circulating levels of VEGF, bFGF, and IL-8 correlate with response, and (2) circulating proangiogenic and proinflammatory cytokines are altered. Local radiation alone has powerful local antiangiogenic effects[32- 36] that can include systemic lowering of circulating angiogenic factors such as bFGF and VEGF. Investigators have demonstrated decreases in VEGF, IL-8, and monocyte chemotactic protein-1 (MCP-1) in tumor after celecoxib administration, probably due to decreased inflammation and thus decreased macrophage activation.[ 37] Among these angiogenic factors, bFGF, VEGF, and IL-8 are easily measured in the circulation and have been markers of tumor aggressiveness and response in breast, melanoma, and bladder cancers.[38-41] Therefore, blood will be collected to measure these factors and determine if the treatment regimen is associated with a decrease in these factors, and if angiogenic factors in the circulation correlate with prognosis. Celecoxib does not appear to sensitize normal tissue to radiation; in fact, it appears to achieve the opposite effect.[18,19] For example, fibrosis and acute inflammation after radiation are significantly reduced in several strains of mice if celecoxib is given orally for at least 5 days near the time of irradiation. The strain differentiation appears to be related to the intrinsic differences in constitutive expression of inflammatory and fibrogenic cytokines, including TGFbeta- 1. TGF-beta-1 has been shown to predict susceptibility to the development of lung fibrosis in patients undergoing bone marrow transplantation and lung irradiation.[42-45] Serum levels of TGF-beta-1, IL-1, and IL-6 will be measured to determine if they correlate with pulmonary toxicity. Laboratory data of COX-2 inhibitors and their role in the treatment of lung cancer are encouraging. This important study will evaluate the effectiveness of celecoxib with concurrent radiation therapy in patients with NSCLC and enhance our knowledge of the mechanisms of action. This patient population, for whom concurrent chemotherapy and radiation may be unnecessarily toxic, is ideal for this therapy as it does not appear to increase locoregional toxicity and may have a protective effect on normal tissue.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article
References:
1. Williams CS, Mann M, Dubois RN: The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18:7908- 7916, 1999.
2. Eberhart CE, Coffey RJ, Radhika A, et al: Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 107:1183- 1188, 1994.
3. Oshima M, Dinchuk J, Kargman SL, et al: Suppression of intestinal polyposis in Apc delta716 knock out mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87:803-809, 1996.
4. Gridelli C, Maione P, Airoma G, et al: Selective cyclooxygenase-2 inhibition and nonsmall cell lung cancer. Curr Med Chem 9(21):1851-1858, 2002.
5. Milas L: Cyclooxygenase-2 (COX-2) enzyme inhibitors as potential enhancers for tumor radioresponse. Semin Radiat Oncol 11(4):290-299, 2001.
6. Hida T, Yatabe Y, Achiwa H, et al: Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res 58:3761- 3764, 1998.
7. Maruta H, Kawano S, Tsuji S, et al: Cyclooxygenase enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol 94:451-455, 1999.
8. Sheehan KN, Sheahan K, O’Donoghue DR, et al: The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA 282:1254-1257, 1999.
9. Achiwa H, Yatabe Y, Hida T, et al: Prognostic significance of elevated cyclooxygenase- 2 expression in primary resected lung adenocarcinomas. Clin Cancer Res 5(5):1001-1005, 1999.
10. Soslow RA, Dannenberg AJ, Rush D, et al: Cox-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 89:2637-2645, 2000.
11. Wolff H, Saukkonen K, Anttila S, et al: Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res 58(22):4997-5001, 1998.
12. Hida T, Kozaki K, Muramatsu H, et al: Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res 6:2006-2011, 2000.
13. Sheng H, Shao J, Kirkland SC, et al: Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest 99:2254-2259, 1997.
14. Masferrer JL, Leahy KM, Koki AT, et al: Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res 60:1306-1311, 2000.
15. Leahy KM, Ornberg RL, Wang Y, et al: Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res 62:625-631, 2002.
16. Nakata H, Uemura Y, Kobayashi M, et al: Cyclooxygenase-2 inhibitor NS-398 suppresses cell growth and constitutive production of granulocyte-colony stimulating factor and granulocyte macrophage-colony stimulation factor in lung cancer cells. Cancer Sci 94(2):173-180, 2003.
17. Steinauer KK, Gibbs I, Ning S, et al: Radiation induces upregulation of cyclooxygenase-2 (COX-2) protein in PC-3 cells. Int J Radiat Oncol Biol Phys 48(2):325- 328, 2000.
18. Kishi K, Petersen C, Hunter N, et al: Preferential enhancement of tumor radioresponse by a cyclooxygenase-2 inhibitor. Cancer Res 60:1326-1331, 2000.
19. Milas L, Kishi K, Hunter N, et al: Enhancement of tumor response to gamma-radiation by an inhibitor by cyclooxygenase-2 enzyme. J Natl Cancer Inst 91:1501-1504, 1999.
20. Anscher MS, Kong FM, Marks LB, et al: Changes in plasma transforming growth factor beta during radiotherapy and the risk of symptomatic radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys 37:253-258, 1997.
21. Milas L, Hanson WR: Eicosanoids and radiation. Eur J Cancer 31A:1580-1585, 1995.
22. Dillman RO, Hernodn J, Seagren SL, et al: Improved survival in stage III non-small cell lung cancer: Seven-year follow-up of Cancer and Leukemia Group B (CALGB) 8433 trial. J Natl Cancer Inst 88(17):1210-1215, 1996.
23. Sause WT, Scott C, Taylor S, et al: Radiation Therapy Oncology Group (RTOG) 88- 08 and Eastern Cooperative Oncology Group (ECOG) 4588: Preliminary results of a phase III trial in regionally advanced, unresectable non-small cell lung cancer. J Natl Cancer Inst 87(3) 198-205, 1995.
24. Curran WJ, Scott C, Langer C, et al: Phase III comparison of sequential vs. concurrent chemoradiation for patients with unresected stage III non-small cell lung cancer: Report of Radiation Therapy Oncology Group (RTOG) 9410. Lung Cancer 29:93, 2000.
25. Furuse K, Fukuoka M, Kawahara M, et al: Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 17:2692-2699, 1999.
26. Lau DH, Ryu JK, Gandara DR: Chemoradiotherapy for poor-risk stage III nonsmall cell lung cancer. Semin Oncol 24(4 suppl 12):S12-110–S12-112, 1997.
27. Firat S, Bousamra M, Gore E, et al: Comorbidity and KPS are independent prognostic factors in stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 52(4):1047-1057, 2002.
28. Firat S, Byhardt R, Gore E: Comorbidity and KPS are independent prognostic factors in stage III non-small cell lung cancer (NSCLC): An institutional analysis of patients treated on 4 RTOG studies. Int J Radiat Oncol Biol Phys 54(2):357-364, 2002.
29. Extermann M, Overcash J, Lyman GH, et al: Comorbidity and functional status are independent in older cancer patients. J Clin Oncol 16:1582-1587, 1998.
30. Montazeri A, Milroy H, Hole D, et al: Quality of life in lung cancer patients: As an important prognostic factor. Lung Cancer 31(2- 3):233-240, 2001.
31. Inouye SK, Peduzzi PN, Robison JT, et al: Importance of functional measures in predicting mortality among older hospitalized patients. JAMA 279:1187-1193, 1998.
32. Okunieff P, Barrett JA, Phang SE, et al: Circulating basic fibroblast growth factor declines during Cy/TBI bone marrow transplantation. Bone Marrow Transplant 23:1117-1121, 1999.
33. Urano M, Tanaka N: The effect of preirradiated tumour bed on the response of a murine fibrosarcoma to elevated temperatures. Int J Hyperthermia 5:617-624, 1989.
34. Okunieff P, Dols S, Lee J, et al: Angiogenesis determines blood flow, metabolism, growth rate, and ATPase kinetics of tumors growing in an irradiated bed: 31P and 2H nuclear magnetic resonance studies. Cancer Res 51:3289-3295, 1991.
35. Okunieff P, Urano M, Kallinowski F, et al: Tumors growing in irradiated tissue: Oxygenation, metabolic state, and pH. Int J Radiat Oncol Biol Phys 21:667-673, 1991.
36. Penhaligon M, Courtenay VD, Camplejohn RS: Reduction of tumor bed effect after angiogenic stimulation. Br J Radiol 61:1168-1171, 1988.
37. Hida T, Yatabe Y, Achiwa H, et al: Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res 58:3761- 3764, 1998.
38. Ramsay J, Zietman A, Preffer F, et al: The growth and cell kinetics of a secondary tumor after radiation or surgical treatment of the primary tumor. Int J Radiat Oncol Biol Phys 17:809-813, 1989.
39. Weidner N, Folkman J, Pozza F, et al: Tumor angiogenesis: A new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 84:1875- 1887, 1992.
40. Weidner N, Semple JP, Welch WR, et al: Tumor angiogenesis and metastasis: Correlation in invasive breast carcinoma. N Engl J Med 324:1-8, 1991.
41. Nguyen M, Watanabe H, Budson AE, et al: Elevated levels of the angiogenic peptide basic fibroblast growth factor in urine of bladder cancer patients. J Natl Cancer Inst 85:241- 242,1993.
42. Anscher MS, Kong FM, Marks LB, et al: Changes in plasma transforming growth factor beta during radiotherapy and the risk of symptomatic radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys 37:253-258, 1997.
43. Anscher MS, Marks LB, Shafman TD, et al: Using plasma transforming growth factor beta-1 during radiotherapy to select patients for dose escalation. J Clin Oncol 19:3758-3765, 2000.
44. Anscher MS, Peters WP, Reisenbichler H, et al: Transforming growth factor β as a predictor of liver and lung fibrosis after autologous bone marrow transplantation for advanced breast cancer. N Engl J Med 328:1592-1598, 1993.
45. Fu XL, Huang H, Bentel G, et al: Predicting the risk of symptomatic radiation-induced lung injury using both the physical and biologic parameters V(30) and transforming growth factor beta. Int J Radiat Oncol Biol Phys 50(4):899-908, 2001.