Irinotecan and 5-FU/Leucovorin in Metastatic Colorectal Cancer: Balancing Efficacy, Toxicity, and Logistics
Variations of fluorouracil (5-FU) therapy have formed the backboneof chemotherapy for advanced colorectal cancer for many years.With the advent of newer agents that often work best with or even requirechemotherapy to optimize their activity, the issue of the optimalschedule and regimen of administration of 5-FU has taken on a renewedimportance. The combination of irinotecan with 5-FU/leucovorinhas consistently improved survival and response rates in comparisonto 5-FU/leucovorin alone. However, the combination also increasesthe toxicity of the treatment, thus resulting in continuing attemptsto improve on the toxicity profile of the combination, while retainingor improving upon the therapeutic outcomes. This article reviewsthe various combinations of irinotecan with 5-FU/leucovorin.
Variations of fluorouracil (5-FU) therapy have formed the backbone of chemotherapy for advanced colorectal cancer for many years. With the advent of newer agents that often work best with or even require chemotherapy to optimize their activity, the issue of the optimal schedule and regimen of administration of 5-FU has taken on a renewed importance. The combination of irinotecan with 5-FU/leucovorin has consistently improved survival and response rates in comparison to 5-FU/leucovorin alone. However, the combination also increases the toxicity of the treatment, thus resulting in continuing attempts to improve on the toxicity profile of the combination, while retaining or improving upon the therapeutic outcomes. This article reviews the various combinations of irinotecan with 5-FU/leucovorin.
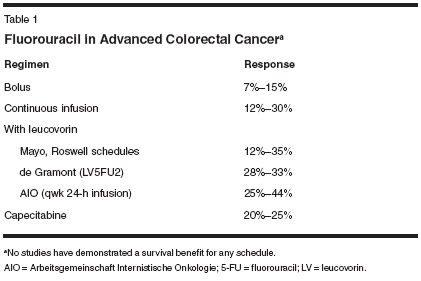
For more than 40 years, attempts to improve upon therapy for metastatic colorectal cancer revolved around variations of administration or modulation of fluorouracil (5-FU), with no regimen or schedule demonstrating clear superiority in terms of survival. Moreover, three phase III studies failed to demonstrate any significant survival benefit with the use of infusional 5-FU (Table 1).[1-7] New Treatment Agents With the advent of new cytotoxic chemotherapeutic agents such as irinotecan (Camptosar),[8-11] oxaliplatin (Eloxatin),[7,12,13] and capecitabine (Xeloda),[14,15] as well as more recently and dramatically, targeted therapies such as cetuximab (Erbitux)[16] and bevacizumab (Avastin),[17] significant advances have been made in the treatment of advanced colorectal cancer over the past decade (Table 2). (Indeed, cetuximab has recently been approved by the US Food and Drug Administration [FDA] for the treatment of patients with epidermal growth factor receptor [EGFR]-positive metastatic disease refractory to irinotecan-based therapy.) However, despite this proliferation of new agents, the optimal combinations and sequences of employing these therapies remain uncertain. One result of the numerous evaluations of the permutations of 5-FU in metastatic colorectal cancer is that there are numerous ways that 5-FU/leucovorin can be combined with these secondgeneration chemotherapies and targeted therapies. For the foreseeable future, the initial management of metastatic colorectal cancer will continue to employ some form of fluoropyrimidine, often 5-FU/leucovorin, as both oxaliplatin and irinotecan in combination with 5-FU/leucovorin are significantly superior to 5-FU/leucovorin alone.[7,10- 12] Furthermore, the registration study of oxaliplatin as second-line chemo- therapy has also clearly demonstrated that oxaliplatin in combination with infusional 5-FU/leucovorin possesses greater antitumor activity than either oxaliplatin or 5-FU/leucovorin alone.[12] However, it remains unclear whether irinotecan- or oxaliplatin-based combination chemotherapy regimens are superior. Studies that have controlled for the fluoropyrimidine regimens suggest that the antitumor efficacy of oxaliplatin- or irinotecan-based regimens are similar.[18] Bevacizumab The efforts to determine the optimal initial chemotherapy combination and schedule have taken on a renewed importance in light of the phase III study by Hurwitz et al.[17] A total of 813 patients with metastatic colorectal cancer and no prior chemotherapy for unresectable metastatic disease were randomly assigned to the combination of irinotecan and bolus 5-FU/leucovorin (IFL) administered weekly for 4 weeks, followed by a 2-week break that was the standard regimen in the United States at the time of study design, with or without the monoclonal antibody targeting vascular endothelial growth factor (VEGF) bevacizumab 5 mg/kg every other week. The addition of the antibody resulted in a significant improvement in response rate (44.8% vs 34.8%; P = .004), median progression-free survival (10.6 vs 6.2 months; P < .001) and median overall survival (20.3 vs 15.6 months; P < .001), in comparison to the then-standard therapy IFL. This benefit occurred with a relatively modest increase in toxicity, represented primarily by grade 3 (requiring therapy) hypertension (11% vs 2.3%) and intestinal perforation (1.5% vs 0%). The 60-day mortality rates in this study were similar in the two arms (4.5% with IFL and 3.0% with the addition of bevacizumab).[17] An interesting question that has arisen is whether the benefits of bevacizumab are specific to this combination of IFL, or whether they can be extrapolated to other fluoropyrimidine regimens and schedules. Ongoing and completed studies that will be reported in the near future will help answer this question. Therefore, the question of the optimal schedule of IFL, thus the method of administering 5-FU/ leucovorin, remains important. 5-FU/Leucovorin With Irinotecan The FDA approved the combination of irinotecan with 5-FU/leucovorin for use as initial chemotherapy for patients with metastatic colorectal cancer in 2000, based on two studies that clearly demonstrated a survival benefit from adding irinotecan to either bolus or infusional 5-FU/leucovorin.[ 10,11] Two different schedules were approved: IFL and a biweekly regimen of irinotecan on day 1, and leucovorin with a combination of bolus and infusional 5-FU and leucovorin. The IFL regimen found initial favor among US oncologists, apparently because of the ease of administration of bolus 5-FU/leucovorin. Meanwhile, in Europe, the infusional 5-FU schedules had found greater sway, in part because of somewhat greater response rates, and an improved toxicity profile in comparison to bolus 5-FU/leucovorin schedules.
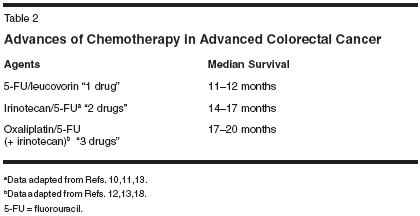
No phase III studies have yet compared these two schedules. A randomized phase II study from Morocco was recently reported in which 154 patients were randomly assigned to IFL, infusional 5-FU/leucovorin (LV5FU2 or "de Gramont schedule") with irinotecan, or alternating irinotecan with bolus 5-FU/leucovorin ("Mayo schedule"). The arms were not intended to be compared directly, but their antitumor activity appeared similar. The toxicity profiles were also similar to the expected side effects, although the infusional 5-FU arm produced somewhat less myelosuppression.[19] IFL Toxicity However, it has become evident that IFL induces significant toxicity. This was highlighted by Rothenberg et al[20] in a 2001 review of toxic deaths occurring within 60 days of the initiation of treatment. The results were obtained from the North Central Cancer Treatment Group (NCCTG) study 9741 (4.8% of 289 patients) in metastatic disease, and the Cancer and Leukemia Group B (CALGB) study 89803 (2.2% of 635 patients) evaluating the potential role of IFL as adju- vant therapy for patients with stage III colorectal cancer. These results could be compared to 0.8% 60-day mortality with bolus 5-FU/leucovorin as adjuvant therapy in patients with stage III colorectal cancer, and 1.8% with oxaliplatin with infusional 5-FU/ leucovorin (FOLFOX4) in metastatic cancer. This report divided the predominant toxicities of IFL into several syndromes. The first is a gastrointestinal syndrome, comprising diarrhea, nausea, vomiting, or abdominal cramping, often in the setting of neutropenia, dehydration, and electrolyte abnormalities. The second is a vascular syndrome that included myocardial infarctions, pulmonary emboli, and strokes. The former syndrome caused deaths in about 2% of all patients treated, and the latter about 1%.[20] As a result of these findings, this regimen started to fall somewhat from favor. From the registration study and NCCTG 9741, the toxicities of IFL were similar. Severe neutropenia was reported in 40% to 54% of patients, diarrhea in 22% to 28%, and nausea/ vomiting in 9% to 16%.[10,13] Further questions about the role of IFL have been compounded by the findings of NCCTG 9741 in which Goldberg et al demonstrated that FOLFOX4 was superior to IFL as initial chemotherapy in metastatic colorectal cancer.[13]
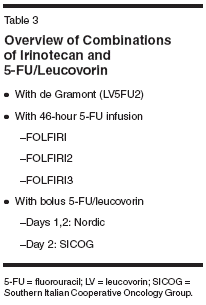
Irinotecan With LV5FU2 (DeGramont) Schedule As a result of the difficulties with the toxicities from the traditional IFL schedule and the approval of oxaliplatin/ 5-FU/leucovorin as second-line chemotherapy, infusional schedules have garnered greater attention and acceptance in the United States. A review of the studies evaluating infusional 5-FU/leucovorin with irinotecan demonstrated that the mortality rate with this schedule was less than 1%, compared to 2.2% to 4.8% with IFL.[11] To date, the most thoroughly studied regimen of infusional 5-FU/leucovorin with irinotecan in phase III studies is in combination with LV5FU2, or the de Gramont regimen. Douillard et al treated 145 patients with this combination as first-line chemotherapy for metastatic disease. Although neutropenia remained the most commonly noted severe toxicity (46% of patients), severe diarrhea (13%) and vomiting (3%) were less frequently reported than with IFL.[11] Given the potential for pharmacogenetic differences between Asians and Europeans that comprised the populations in most of the reported studies with irinotecan and 5-FU/leucovorin, a report on this regimen by Tai et al is interesting. They reported that in an Asian population, this regimen was similarly well tolerated in 18 patients, with severe leukopenia or diarrhea each in 8% of cycles, and severe stomatitis in 3% of cycles. In these patients who received the therapy as second-line therapy after prior oxaliplatin, four (22%) had partial responses, with a median survival of 7.5 months.[21] An interesting modification of this regimen has been explored by a group of Italian investigators. They administered the dose of irinotecan as 90 mg/m2 on each day. When evaluated in 54 patients in the initial therapy setting, the regimen demonstrated similar antitumor activity as other schedules of IFL, as determined by objective responses (41%, including 7% complete responses) and a median time to progression of 8.7 months, a median survival of 18.8 months, and a 1-year survival of 74%. Severe toxicities were relatively uncommon: grade 3/4 neutropenia was reported in 17% of patients, severe diarrhea, asthenia, and thrombocytopenia were each seen in 6% of patients, and 4% of patients experienced grade 3 or 4 nausea/vomiting or anemia.[22] FOLFIRI Regimen However, administering LV5FU2 with irinotecan is logistically burdensome to both the patient and physician, and the development of alternative schedules has been of great interest. (Table 3 presents a listing of combinations of irinotecan and 5-FU/ leucovorin.) The best-explored variant of the LV5FU2 schedule with irinotecan is FOLFIRI. This regimen also involves a biweekly infusion of irinotecan at 180 mg/m2 over 90 minutes and leucovorin at 400 mg/m2, followed by a 400-mg/m2 bolus of 5-FU, then a 46-hour infusion of 5-FU at a dose of 2,400 to 2,800 mg/m2. This regimen was first reported by Andre et al,[23] and was initially evaluated as a second-line regimen, after the failure of oxaliplatin-based therapy. While it induced an objective response in only 6% of the 33 patients, it resulted in a median survival of 43 weeks. Toxicity was modest, with severe neutropenia in only 15% of patients and grade 3/4 diarrhea and nausea/vomiting in 12% and 15%, respectively.[ 23] These efficacy and toxicity results were confirmed by British investigators using the regimen in both the first- and second-line setting. Leonard et al reported a response rate of 30% in 43 patients receiving FOLFIRI as initial chemotherapy, and 23% in the 36 patients who were treated with FOLFIRI after prior fluoropyrimidine therapy.[24] FOLFIRI vs FOLFOX This schedule has also been the focus of a French phase III study reported by Tournigand et al,[18] as first-line therapy, compared to FOLFOX6. At the time of progression, patients initially treated with FOLFIRI subsequently received FOLFOX6, and those who were given FOLFOX6 as initial chemothera- py then received FOLFIRI. In this setting, FOLFIRI yielded responses in 56% of 109 patients, and a median time to progression of 8.6 months, results that were similar to those with FOLFOX6 (response rate of 54%, median time to progression of 8.0 months). Of the 111 patients who received first-line FOLFOX6, 68 then received FOLFIRI as second-line therapy upon the diagnosis of progressive disease. As second-line therapy in this study, FOLFIRI was somewhat disappointing, producing an objective response in 4% of patients and a median progression- free survival of 2.5 months. Overall, the median survival for patients in the two arms was similar: 21.5 months with initial FOLFIRI and 20.6 months with second-line FOLFIRI. As expected, FOLFIRI was well tolerated as both first- and secondline therapy. Severe neutropenia was reported in 24% of patients treated with FOLFIRI as initial therapy and 21% treated with second-line FOLFIRI. Grade 3/4 nonhematologic toxicity was also relatively infrequent: diarrhea (14%/8%), nausea/vomiting (13%/3%), and mucositis (10%/3% of patients who received FOLFIRI as first- and second-line therapy, respectively). A major concern with IFL is the toxicity of the regimen, as reflected by the 60-day mortality rates. As first- and second-line therapy, FOLFIRI yielded 60-day mortality rates of 4% and 3%, respectively, which were comparable to those for FOLFOX6 (3% as first-line therapy and 4% as second-line therapy) in this study.[18] Modifications to FOLFIRI Although FOLFIRI is a promising regimen, French investigators have undertaken several modifications to attempt to improve further the combination. The FOLFIRI2 regimen sought to evaluate the value of changing the timing of the interaction between 5-FU and irinotecan by delivering the latter after the completion of the 5-FU infusion. Further modulation of 5-FU was attempted with the addition of 1,500 mg of hydroxyurea daily during therapy. Not surprisingly, increases in severe neutropenia (52% of 29 patients), diarrhea (31%), nausea (17%), and mucositis (14%) were reported. Responses were reported in 17% of patients, and the median survival of patients was 42 weeks.[25] Since the antitumor efficacy did not appear superior to FOLFIRI despite greater toxicity, further investigation of this regimen has not been undertaken. More recently, the same investigators created the FOLFIRI3 regimen, in which patients receive 100 mg/m2 of irinotecan both prior to and after the 46-hour 5-FU infusion that has been reduced to a dose of 2,000 mg/m2. The bolus 5-FU and the leucovorin were eliminated. A pair of reports (initially from a single institution and more recently a multi-institutional study) have consistently reported a 20% response rate in second-line therapy after oxaliplatin and infusional 5-FU. This regimen was fairly well-tolerated: severe neutropenia (30% to 35% of patients), diarrhea (15% to 19%), and mucositis in (5% to 7.5%).[26,27] A potential concern with this schedule is based on data from Falcone et al, who conducted a study evaluating the impact of sequencing irinotecan and a 48-hour infusion of 5-FU. Their results suggested that administration of irinotecan prior to 5-FU is better tolerated, with less diarrhea, nausea/ vomiting, myelosuppression, and stomatitis than the sequence of 5-FU followed by irinotecan.[28] However, the FOLFIRI3 regimen suggests that there is no significant difference in toxicity compared to a schedule where irinotecan is administered only before 5-FU. The AIO Schedule A weekly 24-hour infusion of 5-FU, with leucovorin, has been extensively evaluated by the German Cancer Society Arbeitsgemeinschaft Internistische Onkologie (AIO). Vanhoefer et al performed a dose-escalation study adding weekly irinotecan. They recommended for further investigation a dose of irinotecan at 80 mg/m2 and 5-FU at 2,600 mg/m2 over 24 hours and leucovorin at 500 mg/m2 weekly for 6 weeks, followed by a 1-week break. The prima- ry toxicity encountered on this schedule was diarrhea, with little myelosuppression.[ 29] Based on the European patterns of 5-FU/leucovorin use, as well as the results from this phase I study, this combination has undergone further evaluation. In their phase III study evaluating infusional 5-FU/leucovorin with or without irinotecan, Douillard et al also treated a group of patients with the German AIO schedule. The 54 patients treated with this regimen also experienced frequent severe neutropenia (29%), diarrhea (44%), vomiting (11%), and asthenia (7%).[11] To clarify the benefits of the addition of irinotecan to the weekly infusional AIO schedule of 5-FU/ leucovorin, Kohne et al[30] and the European Organisation for Research and Treatment of Cancer conducted an additional phase III study (EORTC 40986). A total of 430 patients with metastatic colorectal cancer without prior chemotherapy for metastatic disease were randomly assigned to the AIO 5-FU (2,600 mg/m2 over 24 hours)/leucovorin (500 mg/m2 over 2 hours) weekly for 6 weeks, with cycles every 7 weeks, or 5-FU/leucovorin (500 mg/m2 over 2 hours) on the AIO schedule, with a dose reduction in 5-FU/leucovorin, in combination with irinotecan (80 mg/m2 over 30 minutes). In the experimental arm, the initial dose of infusional 5-FU was 2,300 mg/m2 over 24 hours. The incidence of severe diarrhea in these patients was concerning, occurring in 36% of patients, and the dose was subsequently reduced to 2,000 mg/m2. As a result of this protocol change, the occurrence of severe diarrhea decreased to 24%, which was comparable to that with 5-FU/leucovorin in the control arm (21%). Aside from a slight increase in the frequency of grade 3/4 neutropenia (7% with irinotecan, vs 3%), other severe toxicities were similar in the two arms, including severe nausea/vomiting in 9% of patients, and stomatitis in 3% in those receiving irinotecan. The toxic death rate (1.9% in each arm) and deaths within 60 days of therapy (2.3% with irinotecan, compared to 3.2% in the control arm) were similar, supporting the hypothesis of an acceptable tolerability of the combination. Moreover, the median relative dose intensity of 5-FU was 81% with irinotecan and 83% without irinotecan; the median relative dose intensity of irinotecan was 79%
Not surprisingly, this study also demonstrated that the addition of irinotecan to 5-FU/leucovorin produced a significantly greater objective response rate (54.2% vs 31.8%; P = .0001), and met its primary objective of a significant improvement in progression-free survival (8.5 months compared to 6.4 months; P = .0001). However, the improvement in median survival with the addition of irinotecan (20.1 vs 16.9 months; P = .2779 and .0509) was not statistically significant, perhaps because 62% of the patients who received 5-FU/leucovorin alone as initial therapy subsequently received irinotecan.[30] As a result of the findings from this study, the regimen of irinotecan at 80 mg/m2 with at 5-FU 2,000 mg/m2 over 24 hours and leucovorin at 500 mg/m2 weekly for 6 weeks, with cycles repeated every 7 weeks, is now the reference standard for the EORTC. Irinotecan With Longer Infusions of 5-FU Several investigators have also studied irinotecan in combination with longer infusions of 5-FU. In a dose- escalation study, Kakolyris et al administered a 4-day infusion of 5-FU followed by irinotecan on day 4. The recommended dose of 5-FU was 600 mg/m2/d, with 350 mg/m2 of irinotecan, with cycles repeated every 21 days. As first-line therapy, an overall response rate of 22% in 42 patients was noted, with 62% of patients alive with a median follow-up of 13 months. The predominant toxicities reported were diarrhea and neutropenia, with few patients experiencing nausea/ vomiting and mucositis.[31] Also focusing on an extended infusion of 5-FU, Ohtsu et al performed a phase II study of irinotecan at 150 mg/m2 on days 1 and 15, and a 5-day infusion of 5-FU at 600 mg/m2/d from days 3 through 7, with cycles repeated every 28 days. When employed as initial chemotherapy for metastatic disease, the investigators reported a response rate of 45% in 40 patients, with a median survival of 15.9 months, 1-year survival rate of 62.5%, and median progression-free survival of 7.0 months. Again, the main severe toxicities of this regimen were diarrhea (17.5% of patients) and neutropenia (40%). Similarly, grade 3/4 nausea/vomiting (7.5% of patients) and anemia (12.5% of patients) were less commonly experienced. However, three patients discontinued therapy because of toxicity.[ 32] Taken together, these studies do not suggest that the prolonged 5-FU infusion was superior to other infusional 5-FU/leucovorin schedules, and it is not the major focus of further development in combination with irinotecan. Bolus 5-FU/Leucovorin With Irinotecan Attempting to explore further the potential value of bolus 5-FU/leucovorin in combination with irinotecan, and build upon their standard method of administering 5-FU, Glimelius et al[33] and the Nordic group reported a novel regimen, both as IV boluses. Expanding on prior experience, they administered 5-FU at 500 mg/m2, followed by leucovorin at 60 mg/m2 on days 1 and 2, with irinotecan at 210 mg/m2 on day 1 of a fortnightly schedule. Using this regimen as initial therapy in metastatic colorectal cancer, they reported responses in 39% of 74 patients in this phase II study, with a median survival of 15.6 months. The toxicity of this regimen was similar to that seen for IFL. Severe neutropenia occurred in 66% of patients, diarrhea in 16%, vomiting in 11%, and stomatitis in 3%. Only one patient died from toxicity of therapy, from intestinal bleeding.[33] In a similar approach, the Southern Italian Cooperative Oncology Group (SICOG) has also studied a biweekly irinotecan and bolus 5-FU/ leucovorin combination, dubbed IRAFAFU. Irinotecan at 200 mg/m2 was administered on day 1, and on the following day, leucovorin at 250 mg/m2 was infused over two hours, followed immediately by an IV bolus of 5-FU at 850 mg/m2. A total of 118 patients received this combination in a phase II/III study of patients who had not previously received chemotherapy for unresectable metastatic colorectal cancer. The antitumor efficacy of IRAFAFU was similar to that reported with other irinotecan and 5-FU/ leucovorin combinations, with objective responses documented in 36% of patients, including 8% with complete responses, and stabilization of disease or minor response in an additional 21% of patients. The median time to tumor progression was 7.2 months, with a median survival of 14.7 months. The 1-, 2-, and 3-year overall survivals were 60%, 23%, and 11%, respectively. This regimen was fairly well-tolerated, with severe neutropenia report ed in 40% of patients and two cases of neutropenic fever. Severe diarrhea occurred in 13% of patients and severe nausea/vomiting and stomatitis were noted in 3% each.[34] Overall, the antitumor activity and toxicity profile was promising enough for the investigators to name IRAFAFU the new reference standard for the group. Recently, Comella et al reviewed the outcomes with this regimen from this study, focusing on the tolerability and efficacy of the regimen in the elderly population (17 patients who were 70 years of age or older). In the resulting somewhat limited report, they reported no evidence of more severe toxicity or poorer outcomes that occurred in this subgroup of older patients when compared to the others. This led the authors to conclude that IRAFAFU may be a regimen that is suitable for use in otherwise healthy elderly patients.[35]Alternating Irinotecan With 5-FU/Leucovorin Finally, the strategy of alternating irinotecan with 5-FU/leucovorin has been explored by some investigators, with the hope that administering irinotecan and 5-FU/leucovorin separately might decrease the toxicity of the combination while maintaining the antitumor efficacy. The Goldie-Coldman hypothesis postulated that exposing cancer to alternating chemotherapeutic agents that possess different mechanisms of action may further enhance the cytotoxicity of therapy. Although this hypothesis has not been proven in studies with small-cell lung cancer, the potential of the concept has been an impetus for studies of alternating irinotecan and 5-FU/leucovorin. The most-investigated alternating schedule is irinotecan at 350 mg/m2 on day 1 followed by bolus 5-FU (425 mg/m2)/leucovorin (20 mg/m2) on days 22 through 26, with cycles repeated every 6 weeks. Two separate phase II studies have been reported, with the earlier trial by Van Cutsem et al accruing 33 patients.[36] The combination induced objective responses in 30% of patients, and stable disease in almost half. The median survival was 16 months, 1-year survival was 58%, and median progression- free survival was 31 weeks. The authors felt that the safety profile was promising in that only 21% of cycles (albeit 64% of patients) was complicated by grade 3 or 4 neutropenia. Four percent of cycles and 18% of patients experienced neutropenic fever. Interestingly, severe diarrhea or mucositis occurred in 6% and 0.5% of cycles, respectively.[36] A study by Reina et al reported the same response rate and stabilization of disease in 43 patients.[37] The median survival was 18.5 months, and time to progression was 9.0 months. Myelosuppression was also the primary toxicity in this report, with severe neutropenia occurring in only 16% of patients (primarily with 5-FU/leucovorin), and neutropenic fever in only one patient. Severe diarrhea occurred in 2% of cycles and 9% of patients, mainly with the irinotecan. Grade 3/4 mucositis was reported in 4% of cycles and 14% of patients, primarily with 5-FU/leucovorin. Severe nausea/vomiting was noted in 2% of cycles, and 9% of patients when treated with irinotecan.[37] A larger randomized phase II study has also been reported by Bouzid and colleagues.[19] Twenty-six percent of 50 patients had an objective response, all partial responses, and an additional 38% of patients had stabilization of disease. The side-effect profile of the regimen that was reported in this study was somewhat different from the initial report, with severe neutropenia occurring in 22% of patients, with one patient dying from neutropenic fever. Severe diarrhea was reported in 22% of patients, and nausea/ vomiting or stomatitis in 8% and 6% of patients.[19] An experimental regimen of four weekly infusions of 5-FU (2,600 mg/m2 over 48 hours, for 4 weeks)/leucovorin (150 mg/m2) followed by irinotecan (350 mg/m2 on day 36) with cycles repeated every 8 weeks has also been studied. The results were similar to that reported with other schedules and regimens, including an objective response rate of 37% in 37 patients, and median survival of 18.5 months, 1-year survival of 68%, and median time to progression of 8 months. Severe neutropenia was the primary toxicity, occurring in 43% of patients with irinotecan only, including five experiencing neutropenic fever. Fourteen percent of patients experienced severe diarrhea with irinotecan, and an additional 11% with 5-FU. Severe mucositis was noted in 9% of patients with 5-FU.[38] Overall, regimens that alternate irinotecan and 5-FU appear to have demonstrated similar antitumor activity as other combinations of the drugs, but without any clear advantage in terms of toxicity or ease of administration. Modified IFL A less burdensome method of administering irinotecan with 5-FU/leucovorin involved modifying the schedule of IFL. One proposed manner was simply to lower the doses of irinotecan and 5-FU by 20%; this was undertaken by the NCCTG as they completed trial 9741. There did not appear to be a significant diminution of antitumor activity by doing so. Similarly, while conducting an Eastern Cooperative Oncology Group (ECOG) study (E2200) of IFL in com-bination with bevacizumab at 10 mg/kg every other week, Giantonio et al[39] decreased the dose of irinotecan and 5- FU by 20% to 100 mg/m2 and 400 mg/m2. The first 20 patients who enrolled in the study received full doses of IFL, while the remainder was administered the lower doses. Interestingly, the authors reported no statistically significant differences in toxicities between the two groups. Eighteen percent of patients experienced severe diarrhea, and 36% had grade 3 or 4 neutropenia, with four cases of neutropenic fever. The antitumor efficacy of the two schedules also appeared similar, with objective responses in one-third of the 12 evaluable patients who received the full dose of IFL, and 28 of the 58 (48.3%) of those who received the lower dose.[39]
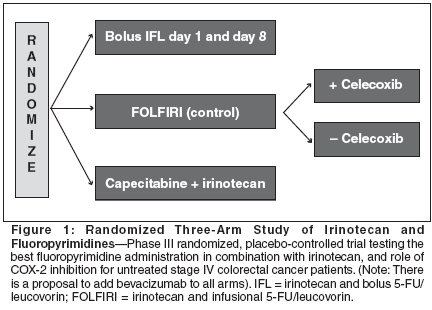
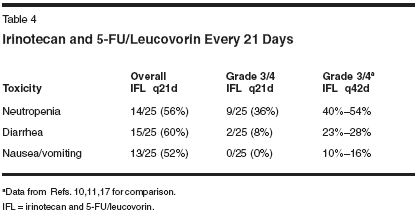
Another logical modification of IFL is to change the schedule from weekly for 4 weeks followed by a 2-week break, to a 2-on, 1-off schedule. The primary rationale for doing this is that during treatment with IFL, the third and especially the fourth weekly doses of therapy were omitted (in about 40% of patients) due to toxicity. This resulted in a decrease in the median relative dose intensity of irinotecan and 5-FU/leucovorin to 72% and 71%, respectively. Considering the difficulties of dose delivery and toxicity in weekly IFL according to the Saltz schedule, which appeared to be cumulative within a cycle, one manner of improving the therapeutic index of weekly IFL would appear to be creation of a break after the second week of therapy, prior to resuming IFL. Our group reviewed the 25 patients with advanced colorectal cancer that have been treated with weekly IFL at the Lombardi Cancer Center at Georgetown University Medical Center. However, therapy was administered on days 1 and 8 every 21 days. For patients who were 75 years or older, the initial dose of irinotecan was 100 mg/m2. The planned dose intensity of this sched- ule would be identical to the Saltz schedule of IFL. None of the patients had received prior chemotherapy. All patients had a good performance status (ECOG 0 or 1). The median age of the population was 57 years (range: 38 to 77 years), including two patients who were 75 years or older. Fifteen of the patients were males. Fifteen of the patients received chemotherapy as adjuvant treatment, and only ten of these patients were administered therapy as treatment for measurable metastatic disease. Five of the patients had stable disease, and the other five had progression of disease on their follow-up evaluation. This schedule was well tolerated, with grade 3/4 neutropenia occurring in nine (36%) patients, and severe diarrhea in only two (8%) (Table 4). Two patients experienced each one episode of febrile neutropenia with the first cycle of therapy, but both tolerated continued treatment with IFL on the 21-day schedule after a 25% dose reduction. One patient was hospitalized with a small bowel obstruction that was believed related to progressive disease; it resolved with conservative therapy. No other grade 3/4 toxicities were noted. These results, especially with regard to the ability to deliver a high dose intensity of the regimen with a simple modification of the schedule of administration, support the hypothesis that altering the schedule of therapy improves the toxicity of IFL. The question of the antitumor activity remains unanswered at this time because of the small patient population, many of whom received the treatment in the adjuvant setting. However, because of the potentially confounding differences between study populations, the comparison of median relative dose intensity between these two study groups requires confirmation in a prospective randomized study. Furthermore, the change in the schedule may not be the only, or primary, reason for the ability to deliver such a high proportion of the intended dose. In particular, the patient population must be considered to be favorable. First, the median age of the treated patients was 57 years, with only two patients being over 75. Moreover, as many of the patients who were treated in this report received the regimen as adjuvant therapy, they may have been a "healthier" population overall. Ultimately, the antitumor activity and tolerability of the 21-day schedule of IFL, then, can only be assessed in the context of a prospective randomized trial. The newest, and logistically most simple, way to combine irinotecan with a fluoropyrimidine is to employ an oral agent such as capecitabine.[40- 43] However, since both agents are metabolized by the liver and have some overlapping toxicities, such as diarrhea, nausea, vomiting, and stomatitis, the optimal doses of the agents for combination therapy remains uncertain. Whether such a combination is truly similar to irinotecan or oxaliplatin in combination with 5-FU/leucovorin combinations is unknown, and awaits the results of randomized phase III studies. These questions regarding the preferred irinotecan and fluoropyrimidine schedule will hopefully be answered by an ongoing industrysponsored study (Figure 1). Discussion and Conclusion Despite the advances in the treatment of patients with advanced colorectal cancer over the past several years, therapy for this common disease, the second leading cause of cancer related mortality in the United States, remains palliative. No prospective randomized studies are currently available to guide physicians and patients toward the optimal sequence and schedules of chemotherapy in this disease. This conundrum especially applies to the determination of the schedule of irinotecan and 5-FU/leucovorin in metastatic colorectal cancer. This is highlighted by the fact that the two regimens that have been accepted by the FDA as reference standards employ different schedules of both irinotecan and 5-FU/leucovorin. As a result of the different schedules of these drugs, a distinct spectrum of toxicities may be observed. With the IV bolus 5-FU/leucovorin schedule that was more commonly used in the United States as part of the IFL schedule, neutropenia and diarrhea appear to predominate. However, with the infusional 5-FU schedules that are more widely utilized in France and Germany, the incidence of neutropenia appears to be less common, although gastrointestinal toxicities remain somewhat prominent. In compensation for what seems to be a decrease in toxicity with an infusional schedule of 5-FU/leucovorin is the logistical difficulty inherent with an ambulatory pump. Since none of these options ideally balances these considerations yet, modifications of these regimens continue. Some of these modifications include the logistically easier-to-employ bolus 5-FU/ leucovorin, and suggest that these newer schedules are relatively well tolerated. The true therapeutic indices of these regimens await comparisons in randomized studies, not merely single-arm studies. These questions of antitumor activity, toxicity of regimens, and burden on patients have become more pressing as newer agents necessarily become integrated in the therapy of advanced colorectal cancer. This urgency of these issues grows with the understanding that novel agents such as bevacizumab and cetuximab are more effective in conjunction with chemotherapy than as single agents. Attempting to remedy the deficiency in medical knowledge regarding the optimal way of employing fluorpyrimidines with newer agents, two studies are investigating this question- one each with irinotecan and oxaliplatin. Pending the long-overdue answers from these studies, when making treatment decisions amongst therapies with similar efficacy, physicians in concert with their patients must determine the individual's preference in terms of balancing toxicity and the logistics of delivering therapy.
Disclosures:
Dr. Hwang has acted as an investigator for and has privately held stock from Pfizer; he has acted as a consultant and investigator for and served on the speaker’s bureau for Sanofi-Synthelabo; he has acted as a consultant for and served on the speaker’s bureau for Genentech; and he has acted as an investigator for and served on the speaker’s bureau for Roche.
References:
1. The Meta-Analysis Group in Cancer: Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol 16:301-308, 1998.
2. Buroker TR, O’Connell MJ, Wieand HS, et al: Randomized comparison of two schedules of fluorouracil and leucovorin in the treatment of advanced colorectal cancer. J Clin Oncol 12:14-20, 1994.
3. Lokich JJ, Ahlgren JD, Gullo JJ, et al: A prospective randomized comparison of continuous infusion fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma: A Mid-Atlantic Oncology Program Study. J Clin Oncol 7:425-432, 1989.
4. Weinerman B, Shah A, Fields A, et al: Systemic infusion versus bolus chemotherapy with 5-fluorouracil in measurable metastatic colorectal cancer. Am J Clin Oncol 15:518-523, 1992.
5. Rougier P, Paillot B, LaPlanche A, et al: 5-Fluorouracil (5-FU) continuous intravenous infusion compared with bolus administration, final results of a randomised trial in metastatic colorectal cancer. Eur J Cancer 33:1789-1793, 1997.
6. Kohne CH, Schoffski P, Wilke H, et al: Effective biomodulation by leucovorin of highdose infusional fluorouracil given as a weekly 24-hour infusion: Results of a randomized trial in patients with advanced colorectal cancer. J Clin Oncol 16:418-426, 1998.
7. De Gramont A, Bosset J-F, Milan C, et al: Randomized trial comparing monthly low dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: A French Intergroup study. J Clin Oncol 15:808-815, 1997.
8. Cunningham D, Pyrhonen S, James RD, et al: Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 352:1413-1418, 1998.
9. Rougier P, Van Cutsem E, Bajetta E, et al: Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure with metastatic colorectal cancer. Lancet 352:1407-1410, 1998.
10. Saltz LB, Cox JV, Blanke C, et al: Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905-914, 2000.
11. Douillard JY, Cunningham D, Roth AD, et al: Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicenter randomized trial. Lancet 355:1041- 1047, 2000.
12. Rothenberg ML, Oza AM, Bigelow RH, et al: Superiority of oxaliplatin and fluorouracil- leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: Interim analysis of a phase III trial. J Clin Oncol 21:2059-2069, 2003.
13. Goldberg RM, Morton RF, Sargent DJ, et al: A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23-30, 2004 (e-pub December 9, 2003).
14. Hoff PM, Ansari R, Batist G, et al: Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: Results of a randomized phase III study. J Clin Oncol 19:2282-2292, 2001.
15. Van Cutsem E, Twelves C, Cassidy J, et al: Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: Results of a large phase III study. J Clin Oncol 19:4097- 4106, 2001
16. Saltz LB, Rubin M, Hochster H, et al: Cetuximab (IMC-C225) plus CPT-11 is active in CPT-11 refractory colorectal cancer that expresses epidermal growth factor receptor (EGFR) (abstract 7). Proc Am Soc Clin Oncol 20:3a, 2001.
17. Hurwitz H, Fehrenbacher L, Novotny W, et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335-2342, 2004.
18. Tournigand C, Andre T, Achille E, et al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol 22:229- 237, 2004 (e-pub December 2, 2003).
19. Bouzid K, Khalfallah S, Tujakowski J, et al: A randomized phase II trial of irinotecan in combination with infusional or two different bolus 5-fluorouracil and folinic acid regimens as first-line therapy for advanced colorectal cancer. Ann Oncol 14:1106-1114, 2003.
20. Rothenberg ML, Meropol NJ, Poplin EA, et al: Mortality associated with irinotecan plus bolus fluorouracil/leucovorin: Summary findings of an independent panel. J Clin Oncol 19:3801-3807, 2001.
21. Tai C-J, Liu J-H, Chen W-S, et al: Irinotecan (CPT11) plus high-dose 5-fluorouracil (5-FU) and leucovorin (LV) as salvage therapy for metastatic colorectal cancer (MCRC) after failed oxaliplatin plus 5-FU and LV: A pilot study in Taiwan. Jpn J Clin Oncol 33:136-140, 2003.
22. Recchia F, Nuzzo A, Lalli A, et al: Multicenter phase II study of CPT-11 fractionated over two days with bimonthly leucovorin and 5-fluorouracil in patients with metastatic colorectal cancer. Anticancer Res 23:2903- 2908, 2003.
23. Andre T, Louvet C, Mandrault-Goebel F, et al: CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. Eur J Cancer 35:1343-1347, 1999.
24. Leonard P, Seymour MT, James R, et al: Phase II study of irinotecan with bolus and high dose infusional 5-FU and folinic acid (modified De Gramont) for first- or second-line treatment of advanced or metastatic colorectal cancer. Br J Cancer 87:1216-1220, 2002.
25. Mabro M, Louvet C, Andre T, et al: Bimonthly leucovorin, infusional 5-fluorouracil, hydroxyurea, and irinotecan (FOLFIRI-2) for pretreated metastatic colorectal cancer. Am J Clin Oncol 26:254-258, 2003.
26 Mabro M, Artru P, Flesch M, et al: Irinotecan, 5-fluorouracil infusion and leucovorin, (FOLFIRI-3) in pretreated patients with metastatic colorectal cancer: Results of a multicenter phase II study (abstract 1125). Proc Am Soc Clin Oncol 22:1125, 2003.
27 Maindrault F, Louvet C, Tournigand C, et al: Leucovorin, 5-fluorouracil infusion and irinotecan (FOLFIRI-3) in pretreated patients with metastatic colorectal cancer (abstract 658). Proc Am Soc Clin Oncol 21:165a, 2002.
28 Falcone A, DiPaolo A, Masi G, et al: Sequence effect of irinotecan and fluorouracil treatment on pharmacokinetics and toxicity in chemotherapy-naïve metastatic colorectal cancer patients. J Clin Oncol 19:3456-3462, 2001.
29 Vanhoefer U, Harstrick A, Kohne C-H, et al: Phase I study of a weekly schedule of irinotecan, high-dose leucovorin, and infusional fluorouracil as first-line chemotherapy in patients with advanced colorectal cancer. J Clin Oncol 17:907-913, 1999.
30 Kohne C-H, Van Cutsem E, Wils JA, et al: Irinotecan improves the activity of the AIO regimen in metastatic colorectal cancer: Results of EORTC GI Group study 40986 (abstract 1018). Proc Am Soc Clin Oncol 22:254, 2003.
31 Kakolyris S, Souglakos J, Kouroussis C, et al: A dose finding study of irinotecan (CPT- 11) plus a four-day continuous 5-fluorouracil infusion in advanced colorectal cancer. Oncology 60:207-213, 2001.
32 Ohtsu A, Boku N, Yoshioka T, et al: A phase II study of irinotecan in combination with 120-h infusion of 5-fluorouracil in patients with metastatic colorectal carcinoma: Japan Clinical Oncology Group Study (JCOG9703). Jpn J Clin Oncol 33:28-32, 2003.
33Glimelius B, Ristamaki R, Kjaer M, et al: Irinotecan combined with bolus 5-fluorouracil and folinic acid Nordic Schedule as firstline therapy in advanced colorectal cancer. Ann Oncol 13:1868-1873, 2002.
34 Comella P, Crucitta E, DeVita F, et al: Addition of either irinotecan or methotrexate to bolus 5-fluorouracil and high-dose folinic acid every 2 weeks in advanced colorectal cancer: A randomized study by the Southern Italy Cooperative Oncology Group. Ann Oncol 13:866-873, 2002.
35Comella P, Farris A, LoRusso V, et al: Irinotecan plus leucovorin-modulated 5-fluorouracil IV bolus every other week may be a suitable therapeutic option also for elderly patients with metastatic colorectal carcinoma. Br J Cancer 89:992-996, 2003.
36 Van Cutsem E, Posso C, Starkhamer H, et al: A phase II study of irinotecan alternated with five days bolus of 5-fluorouracil in first line chemotherapy of metastatic colorectal cancer. Ann Oncol 9:1199-1204, 1998.
37 Reina JJ, Aparicio J, Salvador J, et al: A multicenter phase II study of irinotecan (CPT-11) alternated with 5-fluorourcacil and leucovorin as first-line treatment of patients with metastatic colorectal cancer. Cancer Chemother Pharmacol 52:339-345, 2003.
38 Roasati G, Rossi A, Reggiardo G, et al: A Phase II study of irinotecan alternated with a weekly schedule of high-dose leucovorin and 48-hour 5-fluorouracil infusion in patients with metastatic colorectal cancer. Oncology 62:209- 215, 2002.
39 Giantonio BJ, Levy D, O’Dwyer PJ, et al: Bevacizumab (anti-VEGF) plus IFL (irinotecan, fluorouracil, leucovorin) as frontline therapy for advanced colorectal cancer (advCRC): Results from the Eastern Cooperative Oncology Group (ECOG) Study E2200 (abstract 1024). Proc Am Soc Clin Oncol 22:255, 2003.
40 Bajetta E, Di Bartolomeo M, Mariani L, et al: Randomized multicenter Phase II trial of two different schedules of irinotecan combined with capecitabine as first-line treatment in metastatic colorectal carcinoma. Cancer 100:279-287, 2004.
41 Tewes M, Schleucher N, Achterrath W, et al: Capecitabine and irinotecan as first-line chemotherapy in patients with metastatic colorectal cancer: Results of an extended phase I study dagger. Ann Oncol 14:1442-1448, 2003.
42 Delord JP, Pierga JY Dieras V, et al: Dose escalation and pharmacokinetic study of capecitabine (Xeloda) and irinotecan (CPT-11) in gastro-intestinal tumors: Preliminary results (abstract 397). Proc Am Soc Clin Oncol 21:100a, 2002.
43 Jordan K, Grothey A, Kegel T, et al: Phase II trial of capecitabine/irinotecan and capecitabine/oxaliplatin in advanced gastrointestinal cancers. Clin Colorectal Cancer 4:46-50, 2004.