Long-Term Efficacy and Toxicity of the FEC100 Regimen
Adjuvant chemotherapy has been shown to be beneficial in patientswith breast cancer, and anthracycline-containing regimens are more effectivethan non–anthracycline-containing ones. The French AdjuvantStudy Group (FASG) compared FEC100 and FEC50 (fluorouracil[5-FU]/epirubicin [Ellence]/cyclophosphamide [Cytoxan, Neosar])in patients with node-positive breast cancer, with an end point of overallsurvival. After a median follow-up of 10 years, the benefit/risk ratio of theFEC100 regimen in patients with positive axillary nodes is strongly positive.Furthermore, a medicoeconomic study showed that the cost per yearof life saved was very low-approximately 1,000 euros.
Adjuvant chemotherapy has been shown to be beneficial in patients with breast cancer, and anthracycline-containing regimens are more effective than non–anthracycline-containing ones. The French Adjuvant Study Group (FASG) compared FEC100 and FEC50 (fluorouracil [5-FU]/epirubicin [Ellence]/cyclophosphamide [Cytoxan, Neosar]) in patients with node-positive breast cancer, with an end point of overall survival. After a median follow-up of 10 years, the benefit/risk ratio of the FEC100 regimen in patients with positive axillary nodes is strongly positive. Furthermore, a medicoeconomic study showed that the cost per year of life saved was very low-approximately 1,000 euros.
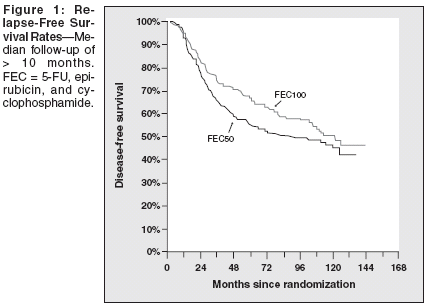
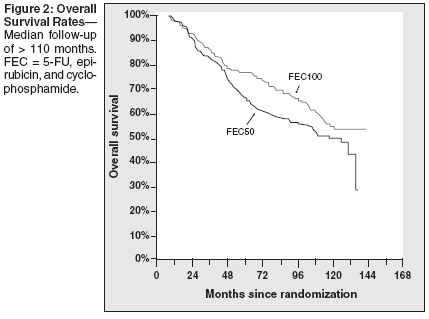
The efficacy of adjuvant chemotherapy has been shown in patients with breast cancer. Furthermore, anthracycline-containing regimens are more effective than non-anthracycline-containing ones.[1] The French Adjuvant Study Group FASG-05 study compared FEC100 and FEC50 (fluorouracil [5-FU]/epirubicin [Ellence]/cyclophosphamide [Cytoxan, Neosar]) in patients with node-positive breast cancer. The main objective of this study was to demonstrate that doubling the epirubicin dose could improve overall survival. The 5-year results[2] have recently been updated to 10 years together with a long-term cardiac and hematologic side effects study[3] and a medicoeconomic study.[4] Clinical Trial Design The main eligibility criteria were female patients younger than 65 years old with histologically proven completely resected breast cancer. All patients had axillary nodal involvement, either more than three positive nodes or between one and three, provided they had no hormone receptors and a histoprognostic grading of II or III. No patients had received prior systemic treatment and all had normal hematologic, hepatic, and cardiac function. A total of 565 patients were randomized between April 1990 and July 1993; 536 were evaluable. No differences were observed in the clinical characteristics of the patients in the two treatment groups. Efficacy Results After a median follow-up of > 110 months, the relapse-free and overall survival rates were higher in those patients receiving FEC100 (P = .03 and .04, respectively); see Figures 1 and 2. At 10 years, the hazard ratios were 1.24 (95% confidence interval [CI] = 1.11-1.36) and 1.29 (95% CI = 1.16- 1.43), respectively. It can be concluded that the 5-year results are maintained at 10 years. The 10-year relapse-free survival rates for the study population were 45.4% ± 3.4 vs 50.7% ± 3.4 for FEC50 and FEC100, respectively (P =.03). The 10-year overall survival rates are very good for a population of patients with such a poor prognosis (50% ±3.3 vs 54.8% ± 3.5 for FEC50 and FEC100, respectively; P =.04 ). Long-Term Side Effects If the short-term side effects in the FEC100 group were more frequent and severe than those in the FEC50 group, they were manageable. Of major concern were the long-term side effects, especially the cardiac toxicity and the risk of leukemia, which could have limited the use of the FEC100 regimen. Cardiac Toxicity
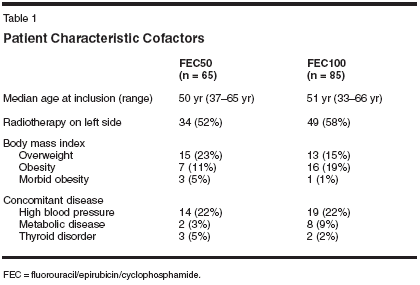
During the first 5 years, 10 cases of cardiac symptoms were observed (6 after FEC50 and 4 after FEC100). In all but two cases (one after FEC50 and one after FEC100), they were observed after retreatment with anthracyclines or anthracenediones at the time of relapse. One patient in the FEC50 group had a myocardial infarction that was considered unrelated to treatment. One patient in the FEC100 group received treatment in spite of a pathologic left ventricular ejection fraction (LVEF) before chemotherapy and should not have been included in the trial. This patient developed congestive heart failure and is still alive, free of disease, but awaiting a heart transplant. Long-Term Cardiac Effects To evaluate the long-term cardiac consequences of epirubicin in patients who had not relapsed (to avoid the interference of a cardiotoxic retreatment) at least 5 years after the end of adjuvant chemotherapy, we carried out a clinical trial in those patients included in the FASG-05 study who had not relapsed and who agreed to a cardiac work-up. Of the 278 patients who were free of disease, 150 could be included. The reasons for noninclusion were as follows: 58 patients refused, 10 relapsed, 15 were lost to follow-up, and 1 died from other causes. In 44 cases, the study was not possible for logistic reasons in some institutions. Cardiac assessment included a clinical visit with a cardiologist, an electrocardiogram, isotopic determination of left ventricular ejection fraction (LVEF), and echocardiography (measurement of LVEF, shortening fraction, telediastolic and telesystolic diameters, isovolumetric relaxation period, and E/ A wave ratio). Patient files showing an abnormality were blindly peer-reviewed by three independent cardiologists who decided whether the cardiotoxicity was related to the adjuvant chemotherapy and whether the side effects were unrelated, possibly related, or probably related according to the associated risk factors. Patient characteristics were the same in both the initial study population (n = 565) and the population included in the cardiac study, as well as in the two groups of the cardiac study (the FEC50 [n = 65] and FEC100 groups [n = 85]). Comorbidities were similar in both groups (Table 1).
Clinical cardiac side effects have been observed in 13 out of 65 (20%) patients in the FEC50 group and in 12 out of 85 (14%) in the FEC100 group. There were two cases of congestive heart failure in the FEC100 group possibly related to treatment. In the FEC50 and FEC100 groups, respectively, there were seven and five cases of dysrythmia, two and one cases of coronary insufficiency, one case in each group of thromboembolic dis ease, and three cases in each group of hypertensive cardiopathy. The panel considered these four cardiac side effects to be unrelated to treatment. Overall, three cases of congestive heart failure have been observed. The first was a decrease of the left ventricular function that was abnormal prior to treatment and occurred early. The second occurred later during follow-up and responded to treatment. Cardiac parameters were normal at cardiac assessment. The third, which was found at the time of cardiac assessment, occurred in a 75-year-old patient with hypertension and responded to treatment.
Grade 1 or 2 National Cancer Institute Common Toxicity Criteria asymptomatic cardiac side effects were observed in 12 patients in the FEC50 group and 35 in the FEC100 group. There were more patients with an abnormal isotopic and/or echocardiographic LVEF in the FEC100 group compared with the FEC50 group (0 and 6 for FEC100 and 0 and 5 for FEC50, respectively); P = .07 and .06. Similarly, the median value of the shortening fraction was lower in the FEC100 group than in the FEC50 group (P = .02). All other parameters were the same. Two years after cardiac assessment, all of these patients remain asymptomatic. Overall, the relationship of cardiac effects to treatment was considered probable in eight cases, possible in nine, and doubtful in three among the FEC100 patients. In conclusion, after a 10-year follow- up when considering cardiac side effects, the risk/benefit ratio is strongly in favor of FEC100. Whether patients with an abnormal LVEF should be treated remains debatable. Other Side EffectsAcute Leukemia
Two cases of acute leukemia have been observed. The first was an acute myeloblastic leukemia type 4 that was diagnosed in a 64-year-old patient 9 months after treatment. The genetic abnormalities were t(8;16) and del(17q21); the patient died 9 months after diagnosis. This leukemia was probably related to treatment. The second case was an acute lymphoblastic leukemia that occurred in a 47-yearold patient 55 months after treatment. A translocation (9;22) was observed. The patient died 1 year after diagnosis; causality is doubtful. Other Cancers
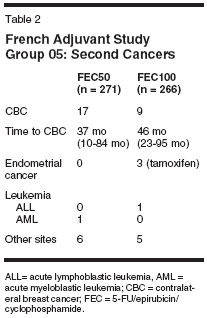
Although the difference was not significant, a contralateral breast cancer has been found less often after FEC100 (9 cases) than after FEC50 (17 cases); see Table 2. Endometrial cancer was observed in three patients receiving tamoxifen (Nolvadex); all patients were in the FEC100 group. The other cancers were found at the same rate in both groups. Ten-Year Event-Free Survival When considering all clinical events, either relapse or long-term side effects, the event-free survival rate was 44.5% in the FEC50 group and 49.3% in the FEC100 group (P = .06). The relative risk of an event was 1.20 (95% CI = 1.08-1.32). Conclusions After a median follow-up of 10 years, the benefit/risk ratio of the FEC100 regimen in patients with positive axillary nodes is strongly positive. Furthermore, a medicoeconomic study showed that the cost per year of life saved was approximately 1,000 euros, which is very low.[4]
Disclosures:
Dr. Bonneterre has served on the speaker’s bureau for Pfizer.
References:
1. Early Breast Cancer Trialists’ Collaborative Group: Polychemotherapy for early breast cancer: An overview of the randomised trials. Lancet 352:930-942, 1998.
2. French Adjuvant Study Group: Benefit of a high dose epirubicin regimen in adjuvant chemotherapy for node positive breast cancer patients with poor prognostic factors: 5-year follow- up results of French Adjuvant Study Group 05 randomized trial. J Clin Oncol 19:602-611, 2001.
3. Bonneterre J, Roché H, Kerbrat P, et al: Long-term cardiac follow-up in relapse-free patients after six courses of fluorouracil, epirubicin, and cyclophosphamide as adjuvant therapy for node-positive breast cancer: French Adjuvant Study Group. J Clin Oncol 22(15):3070-3079, 2004.
4. Bercez C, Bonneterre J, Bonneterre ME, et al: Cost-effectiveness analysis of breast cancer adjuvant treatment: FEC 50 versus FEC 100 (FASG05): Proceedings of the ASCO meeting, Chicago, May 31–June 3, 2003 (abstract 128). J Clin Oncol 22:126, 2003.