Chronic Lymphocytic Leukemia With del(17p13.1): A Distinct Clinical Subtype Requiring Novel Treatment Approaches
This review will discuss the pathophysiology associated with the del(17p13.1) interphase cytogenetic abnormality, the current generally poor outcomes in affected patients, currently approved therapeutic agents, and new agents now undergoing investigation.
Chronic lymphocytic leukemia (CLL) is a very heterogeneous disease with significant variation in clinical presentation, time to disease progression, survival, and aggressiveness of clinical course. A subgroup of patients who have been repeatedly identified as having a poor response to therapy are those with del(17p13.1)-identified by either interphase cytogenetics or other comparable strategies. Although there has been much progress over the past few years in the development of new therapeutic targets for CLL patients, this subgroup has continued to lag behind others. Because of the poor response or significant therapy-related toxicity experienced by patients with del(17p13.1)-and the small number of these patients included in clinical trials-current guidelines are unable to provide suggestions for the care of newly diagnosed, symptomatic but untreated patients (as well as relapsed patients) in this subgroup on account of the modest amount of evidence. However, novel agents are on the horizon that appear to be significantly more effective in this patient population, and these will likely shape the standard of care for these patients in the future.
Introduction
Chronic lymphocytic leukemia (CLL) is a very heterogeneous disease with significant variation in clinical presentation, time to disease progression, survival, and aggressiveness of clinical course. Multiple ongoing laboratory-based studies are attempting to better understand the pathophysiology of this disorder, identify risk factors that portend poor survival, and provide targets for future therapeutic agents. A subgroup of patients who have repeatedly been identified as having a poor response to therapy are those with del(17p13.1) on interphase cytogenetics. Although there has been much progress in CLL therapeutics over the past few years, progress in this patient subgroup has continued to lag behind that seen in other populations. This review will discuss the pathophysiology associated with the del(17p13.1) interphase cytogenetic abnormality, the current generally poor outcomes in affected patients, currently approved therapeutic agents, and new agents now undergoing investigation.
Pathophysiology
FIGURE 1
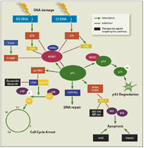
The p53 Pathway
The chemotherapy resistance observed in del(17p13.1) patients is likely related to malfunction of the tumor suppressor protein p53. In humans, the gene that regulates this protein (TP53) is located on the short arm of chromosome 17 (17p13.1).[1] Patients with deletion of 17p have a homozygous TP53 gene, which becomes inactivated by mutation in the vast majority of these patients,[2-4] leading to total lack of function of the p53 pathway (Figure 1). To date, studies have indicated that ~80% of patients with del(17p13.1) also have TP53 gene mutations, and only 4% to 18% of patients studied have TP53 mutations but do not have del(17p13.1).[2,4] However, the region deleted is large, and loss of other genes in this area or associated genomic instability could also be a driver of poor outcomes in these individuals. In response to cellular DNA damage caused by radiation or therapeutics, a normal cell responds by up-regulating the level of p53 protein.[5] Cell-cycle arrest is then induced by p21WAF1 (wild-type p53-activated fragment) through inactivation of cyclin-dependent kinase 2 (CDK2), which blocks the transition of the cell cycle from the G1 phase to the S phase.[6,7] Then cellular and DNA repair enzymes can repair DNA lesions before DNA replication, preventing perpetuation of potentially harmful mutations.[5,8] However, if DNA damage is extensive and irreparable, p53 induces apoptosis of the cell, a process mediated by BAX (BCL-2
[B-cell lymphoma 2]–associated protein X) and down-regulated by BCL-2, and the damaged cell is eliminated.[7,9] The function of p53 is therefore paramount to cellular response to cytotoxic chemotherapies-yet p53 is defective in CLL patients with del(17p13.1) or TP53 mutations.
Epidemiology
With the advent of interphase fluorescence in situ hybridization (FISH) and its heightened diagnostic sensitivity, the del(17p13.1) aberration was detected in ~7% of a large group of mostly untreated patients.[10] Rates of detection of del(17p13.1) following fludarabine therapy have been reported as being as high as 30%,[11] indicating clonal evolution of the disease to withstand chemotherapy. Because the abnormal p53 clones are resistant to chemotherapy, they initiate a negative selection process that slowly increases the number of cells that carry these abnormalities, causing subsequent adverse clinical repercussions.[12] These patients have consistently demonstrated poor survival and a shorter interval from diagnosis to therapy.[7,10,13] The landmark study using interphase FISH for cytogenetic classification of CLL predicted that patients with del(17p13.1) typically require therapy within 1 year of diagnosis and have a meager median overall survival (OS) of only 32 months.[10] Additionally, the landmark trial that established fludarabine, cyclophosphamide, and rituximab (Rituxan) (FCR) as standard-of-care front-line therapy for CLL patients resulted in improved progression-free survival (PFS) and OS when the patients were evaluated all together, but demonstrated that expression of del(17p13.1) was the strongest negative predictive factor for these variables. For the del(17p13.1) patient group, the rate of complete response (CR) was only 5%, 3-year PFS was 18%, and 3-year OS was 38%-compared with rates of 44%, 65%, and 87%, respectively, for the group as a whole.[13] The del(17p13.1) group did not benefit from the addition of rituximab.
In contrast to the majority of historical data, a recent study by Tam et al reviewed 99 treatment-naive (TN) CLL patients with del(17p13.1) and discovered that there is significant clinical heterogeneity within this group. The researchers cautioned against making therapeutic choices solely on the basis of the presence of this cytogenetic abnormality.[14] In the published results of this study, several attempts were made to document this heterogeneity, including by means of short follow-up. Also, patients with del(17p13.1) who also had unmutated IGVH and Rai stage (Rai) ≥ 1 demonstrated worse survival.[14] Another study indicated that a loss of TP53 of ≥ 10% conferred a markedly inferior prognosis, compared with the prognosis in patients with < 10% loss of TP53.[15] Rossi et al used quantitative reverse-transcription polymerase chain reaction (qRT-PCR) to evaluate a series of patients with and without del(17p13.1) cytogenetics.[16] This study identified a microRNA (miR) fingerprint typically expressed in del(17p13.1) patients. It also identified higher levels of miR-21 and low levels of miR-181 in these patients.[16] Many other ongoing research studies attempt to risk-stratify patients within this high-risk group, with the goals of better understanding disease pathology and discovering new and effective therapeutic targets.
Treatment Overview
TABLE
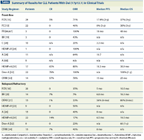
Summary of Results for CLL Patients With Del (17p13.1) in Clinical Trials
As described above and documented in a large number of previous trials, current available treatments have demonstrated discouraging efficacy in the high-risk group of patients who express del(17p13.1). In addition, the small overall number of patients in this group has thwarted progress in the improvement of therapy, with most clinical trials including a very limited number of patients with this cytogenetic feature (Table). In this section of the article, we describe the current guidelines for therapy. In subsequent sections, we describe prior trials and promising agents currently under investigation.
In general, the clinical spectrum of CLL at initial presentation is very heterogeneous. Therefore, a patient usually needs to meet specific criteria prior to initiation of therapy, since some patients can be observed for years without change in clinical condition. Some investigators have proposed that CLL patients who have del(17p13.1) may require earlier therapy because of their known poor prognosis. However, recent trials (described in detail above)[14-16] have suggested heterogeneity even within this group, with some early-stage patients demonstrating a reasonable treatment-free survival. Thus, the indications for therapy in a patient with del(17p13.1) remain the same as for a CLL patient without this deletion. Indications for consideration of treatment that have been established by the International Workshop on Chronic Lymphocytic Leukemia (IWCLL)[17] include the presence of any one of the following:
• Clinical symptoms (fevers, night sweats, weight loss, or painful lymphadenopathy or splenomegaly).
• Cytopenias (hemoglobin < 11 g/dL or platelets < 100 × 1012/L) without other causes.
• Autoimmune hemolytic anemia or thrombocytopenia (idiopathic thrombocytopenia purpura [ITP]) poorly responsive to standard therapy.
• Rapidly progressive disease (lymphocyte count rising to > 300 ×109/L, or rapidly enlarging lymph nodes, spleen, and liver).
Isolated mild thrombocytopenia (platelets 70–100 × 1012/L) can often represent chronic ITP and can be followed closely if no other symptoms are present. A bone marrow biopsy can sometimes help determine whether disease or a combination of disease and ITP is driving this isolated thrombocytopenia.
Once the necessity of treatment has been established, most clinicians turn to the National Comprehensive Cancer Network (NCCN) guidelines for recommendations regarding choice of therapy. Straightforwardly, the NCCN guidelines indicate that there is no set standard-of-care for patients with del(17p13.1) cytogenetics; if eligible, these patients should be enrolled in a clinical trial. Per these guidelines, if a patient achieves a CR or a partial response and is a candidate for transplant, allogeneic stem-cell transplant (alloSCT) should be considered. However, the currency of the NCCN guidelines is limited by the amount of time and strict regulations required to update these recommendations. Research in the field of CLL is constantly bringing new therapeutic options to the fore, and guidelines can become outdated quickly. That being said, the NCCN guidelines are updated as expeditiously as possible based on new published data and represent the most comprehensive and up-to-date source for treatment suggestions. Below, the therapeutic options for patients who are ineligible for a clinical trial will be discussed-for the front-line setting, for the relapsed/refractory (RR) setting, and for the elderly patient population. Figures 2 and 3 depict the treatment algorithms used for patients with del(17p13.1) in the front-line and RR settings, respectively, at our institution.
Treatment of Younger Patients
Chemoimmunotherapy
FIGURE 2
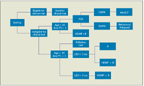
Treatment Algorithm for Patients at Initial Diagnosis of Del(17p13.1) Chronic Lymphocytic LeukemiFIGURE 3
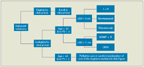
Treatment Algorithm for Patients With Relapsed/Refractory Del(17p13.1) Chronic Lymphocytic
As described in the section on epidemiology, above, standard therapy with FCR is not ideal for this group. However, the CLL-8 trial did demonstrate that del(17p13.1) patients who received front-line FCR (n = 21) had a trend towards an improved overall response rate (ORR) (71% vs 46%, P = .08) and improved 3-year PFS (17.9% vs 0%, P = .052), compared with patients who received fludarabine and cyclophosphamide without rituximab (n = 16), demonstrating a borderline benefit of rituximab in therapy for these patients.[13] Although the ORR trended towards improvement in this group, the 3-year PFS of only 17.9% indicates that the response to FCR is not durable. FCR can therefore be viewed as a cytoreductive therapy for del(17p13.1) patients that is best followed by consolidation with alloSCT or an alternative investigational therapy. In the relapsed setting, the outcomes with FCR in this population are poor. A large study showed a CR of 0% and an ORR of 35%, with a short median PFS (5 months) and median OS (10.5 months) in this population (n = 20),[18] leading to a recommendation against this regimen in relapsed del(17p13.1) patients. Additional studies have been completed with a fludarabine-and-rituximab-only regimen; however, there are insufficient data available to recommend this regimen in the del(17p13.1) group.
When rituximab and bendamustine (Treanda) were combined for therapy of front-line or relapsed CLL, the outcomes in the del(17p13.1) subset were bleak, with a 43% partial response in the front-line patients (n = 3 of 7)[19] and only one patient (7%) responding, and a median PFS of 6.8 months and median OS of 16.3 months in the relapsed population (n = 14).[20] This regimen should not be recommended in this subgroup.
Oxaliplatin (Eloxatin) and cytarabine have been added to fludarabine and rituximab (OFAR) in an attempt to improve outcomes in the setting of relapsed CLL. A trial using this regimen demonstrated an ORR of 33% in fludarabine-refractory CLL patients. The RR del(17p13.1) patients in this trial (n = 15) also demonstrated an ORR of 33%; however, no further subgroup analysis was completed. For the group as a whole, median response duration was 10 months. The main toxicities were hematologic, with a subset of patients not completing therapy due to these adverse effects.[21]
Rituximab and high-dose corticosteroids. Because the addition of rituximab to chemotherapy improved clinical response, and because previous studies had shown response in the relapsed setting to single-agent high-dose methylprednisolone (HDMP),[22] Bowen et al combined rituximab and HDMP for the treatment of relapsed CLL and found an ORR of 56% in patients with del(17p13.1) (n = 9).[23] Subsequently, Castro et al used this regimen in a front-line clinical trial (n = 36); they achieved an ORR of 96% and a median PFS of 30.3 months, with good tolerance of the regimen.[24] Only one patient had del(17p13.1) in this trial, but the HDMP regimen can be used for patients with limited options, who cannot tolerate more aggressive regimens. Patients on this regimen must receive aggressive prophylaxis against opportunistic pathogens, and even with these preventive measures, mortality due to infectious causes is high. Additionally, the metabolic and psychological complications of this regimen can be severe in some patients. Morbidity and mortality are generally more severe in the refractory group of patients receiving this regimen.
Alemtuzumab monotherapy. In light of the success of rituximab, another monoclonal antibody (mAb), alemtuzumab (Campath), which targets CD52, has been extensively studied in this high-risk population-and has shown success. The efficacy of alemtuzumab was initially noted when it was used as a single agent in fludarabine-refractory patients with TP53 mutations and deletions.[25] Following their initial case report, Lozanski et al noted in a larger series of patients a 40% response rate and 8-month median duration of survival in this group,[26] results that were confirmed by several other studies.[11,27] The CAM307 trial, which compared alemtuzumab to chlorambucil, is the only study that provides information on the efficacy of alemtuzumab. The small cohort of patients with del(17p13.1) who received alemtuzumab (n = 11) tended to have a superior ORR (64% vs 20%, P = .08) compared with the ORR in the patients who received chlorambucil (n = 10). However, there was no improvement in PFS, with a disappointing median PFS of 10.7 months in the alemtuzumab group (vs 2.2 months in the chlorambucil group, P = .41); moreover, the patients in the alemtuzumab group experienced more neutropenia and cytomegalovirus reactivation.[28] Another notable limitation of this agent, first recognized in the early studies, was that patients with bulky lymph nodes (> 5 cm) were less likely to respond to the antibody.[29]
Alemtuzumab and high-dose corticosteroids. Using the same rationale as was used in the studies with rituximab and HDMP, and building on the fact that HDMP was previously effective in bulky disease, alemtuzumab was combined with HDMP in a pilot study[30] that led to the multicenter recently published CLL-206 trial. The results of this trial describe the outcomes of both previously untreated (n = 17) and treated (n = 22) CLL patients with del(17p13.1). The regimen showed efficacy-more so in the untreated group-with ORR, CR rate, median PFS, and median OS of 88%, 65%, 18.3 months, and 38.9 months, respectively. (In the previously treated group, these results were 17%, 14%, 6.5 months, and 19.5 months, respectively.) The regimen was fairly toxic, especially in patients over the age of 60, with significant grade 3/4 hematologic and glucocorticoid toxicity (67%) and grade 3/4 infection (51%; 68% in patients over age 60). A 5% treatment-related mortality rate was reported.[31] Similarly, the ongoing CLL-20 trial has combined alemtuzumab with oral dexamethasone (with plans for subsequent maintenance alemtuzumab vs alloSCT) for untreated del(17p13.1), relapsed del(17p13.1), and fludarabine-refractory patients. Preliminary reports have demonstrated ORRs of 100% and 78% and CRs of 23% and 0% in the untreated (n = 31) and relapsed (n = 17) del(17p13.1) patients, respectively. Infection and hematologic toxicity have again been concerns with this regimen.[32]
Multiple additional regimens have been attempts to combine alemtuzumab with various chemoimmunotherapeutic agents (eg, the combination of alemtuzumab with FCR[33, 34]); however, insignificant improvements in response and survival with increased risks of severe infectious toxicity do not allow for recommendation of these therapies in the front-line or relapsed setting.
Ofatumumab. Ofatumumab (Arzerra) is a human mAb to CD20; it is similar to rituximab but binds at a different epitope and causes more potent complement-dependent cytotoxicity than rituximab. Ofatumumab was recently approved in the relapsed population based on a landmark trial in which it was used as a single agent in fludarabine-refractory CLL patients with bulky adenopathy(> 5 cm) (n = 79) and without (n = 59); the resulting ORRs were 47% and 58%, respectively. The del(17p13.1) patients in the group with bulky adenopathy (n = 14) and without (n = 17) had ORRs of 14% and 41%, respectively. No further subgroup analysis was completed; however, PFS for the entire group was slightly less than 6 months. This regimen was very well tolerated, with primarily grade 1 and grade 2 adverse events reported.[35]
Reduced-intensity alloSCT
If a patient is young and otherwise fit and achieves a response with early chemoimmunotherapy regimens, both the NCCN guidelines and the European Group for Blood and Marrow Transplantation consider the presence of del(17p13.1) cytogenetics to be an indication for reduced-intensity alloSCT.[36] A more detailed review on transplantation in this patient group has recently been published.[37] The CLL-3X study indicated that the del(17p13.1) patients (n = 13) who underwent alloSCT demonstrated equivalent outcomes to other cytogenetic groups. The researchers noted a 3-year event-free survival (EFS) of 45% in patients with del(17p13.1), which was consistent with previous studies. Patients with del(17p13.1)who were refractory to chemotherapy at the time of transplant had a significantly shorter EFS (hazard ratio = 2.77; 95% confidence interval = 1.50–5.09).[38] A retrospective study demonstrated a 3-year PFS of 37% for this group (n = 44) and indicated that patients with del(17p13.1) who had had more than three prior therapies had a significantly shorter PFS than those who had received fewer than three prior therapies (19% vs 53%, P = .03).[39]
Although the results of these trials appear promising, it is not clear that the risks of toxicity and transplant-related mortality are balanced by the benefits of prolonged PFS. A prospective trial (Cancer and Leukemia Group B [CALGB] 100701) has been initiated to evaluate this question in previously untreated but symptomatic del(17p13.1) patients. While these trials support the use of transplantation in a young and minimally pretreated population, they cannot be generalized to the typical elderly patient with multiple comorbidities that make pursuit of this modality difficult. All patients with del(17p13.1) who are medically appropriate for reduced-intensity alloSCT should be seen and evaluated by a transplant team early in the course of the disease. For our group, we generally refer such patients at the time we initiate first-line treatment.
Treatment of Elderly Patients
The elderly (> 65 years) subgroup of patients with del(17p13.1) cytogenetics pose a particularly challenging treatment dilemma. Although the median age at diagnosis of CLL is 72 years and ~70% of newly diagnosed patients are older than 65 years,[40] this group has been widely underrepresented in clinical trials, and many of these patients have major medical comorbidities or poor performance status. If an elderly patient has a good performance status, he or she should be enrolled in a clinical trial. If no trial is available, the regimens of alemtuzumab (if no bulky lymph nodes are present) or HDMP + alemtuzumab or HDMP + rituximab could be considered in this group. However, few data exist for alemtuzumab either as monotherapy or in combination with HDMP in the elderly population. A small study (n = 28) using HDMP and rituximab, which included 8 patients over age 70 using this regimen as frontline CLL therapy, demonstrated an ORR of 100% and a CR of 38% in this elderly population; toxicities were minimal. Cytogenetic status was not reported, however.[41] Given the paucity of data in this patient group, clinical studies are needed to better define acceptable and tolerable treatment options. Elderly unfit patients should be referred for palliative care, since there are no therapies for which the benefits outweigh the risks of toxicity.
New Agents
As detailed in this review, standard therapies have had consistently disappointing outcomes in the population of CLL patients with abnormalities of del(17p13.1) or TP53 mutations. The future of this subgroup depends heavily on the development of innovative therapies that act with a p53-independent mechanism. There are several promising therapies currently in clinical trials.
Lenalidomide
Lenalidomide (Revlimid) is an immunomodulatory drug that may work in part by stimulating the host’s own immune system via an increase in the activity of T cells and natural killer cells, which directly induces apoptosis in tumor cells.[42] Additionally, lenalidomide activates CLL cells, making them more recognizable to the immune system; it also increases CD154 expression, thereby activating pro-apoptotic pathways (eg, p73) that can bypass p53.[43] A number of other mechanisms have also been proposed that have recently been reviewed.[44] Eighty RR CLL patients, including patients with high-risk cytogenetic features and bulky lymph nodes, were treated in two phase II studies that demonstrated clinical efficacy. PR was achieved in 29% of the del(17p13.1) patients (n = 14).[45] One of these studies used a higher dose (25 mg/d) for 21 days of a 28-day cycle, but 3 patients experienced severe tumor lysis syndrome (TLS) and 58% had a tumor flare reaction (TFR) (8% had grade 3/4 reactions).[46] The other study used a continuous lower dose (10 mg/d), which resulted in no TLS and TFR in 30%.[47] A subsequent phase I study tested starting at a low dose (2.5 mg/d), with titration up to 20 mg/d to minimize TLS and TFR. In this study, 11.5% (n = 6) achieved PR, and 55.7% (n = 30) achieved stable disease (SD), with a median PFS of ~24 months.[48] Due to lenalidomide’s success, there are ongoing trials evaluating its efficacy as front-line single-agent therapy and in combination with chemoimmunotherapy. One of these studies treated CLL patients over age 65 with lenalidomide and demonstrated clinical efficacy (ORR = 65%, 2-year PFS = 60%) and tolerability in this subset of patients.[49] The six patients with del(17p13.1) did not respond; however, the total number of patients in the study was quite small. Notable efficacy and tolerability have been shown with the combination of lenalidomide and rituximab, both in the front-line[50] and relapsed settings. In the relapsed setting, the ORR of del(17p13.1) patients (n = 15) was 53%, with 13% (n = 2) achieving CR.[51] Registration studies are ongoing with lenalidomide to establish the use of this agent with or without rituximab as one alternative therapy for relapsed del(17p13.1) CLL patients.
B-cell receptor (BCR) antagonists
FIGURE 4
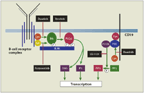
B-Cell Receptor Stimulation
Ibrutinib (formerly PCI-32765) and GS-1101 (formerly CAL-101) are two of the most successful of these new therapies; they have been especially effective in refractory/relapsed CLL (recently reviewed by Woyach et al[52]). These drugs work by targeting the BCR signaling pathway (Figure 4), which has been found to be aberrantly activated in CLL and to promote CLL cell survival and proliferation.[52] Ibrutinib is an oral, irreversible inhibitor of Bruton’s tyrosine kinase (BTK); it promotes apoptosis and inhibits proliferation, migration, and adhesion in CLL cells.[53,54] A phase Ib/II trial evaluated single-agent ibrutinib in RR CLL patients (n = 61) and TN CLL patients (n = 31), all over age 65; the study participants included 24 patients with del(17p13.1) (RR = 22, TN = 2). At the recommended phase I dose (420 mg/d), the ORR was 67% and 73% in the RR and TN groups, respectively, and estimated 12-month PFS was 88% and 93.3%, respectively. An additional 22% of the RR group achieved a nodal response, with residual lymphocytosis. The initial lymphocytosis was likely due to CLL cell mobilization from the bone marrow, and most patients returned to baseline with a break in treatment or longer-term continuation of the drug. The ORR was independent of high-risk factors, including del(17p13.1). The majority of toxicities were less than grade 2, making this a very tolerable as well as an efficacious regimen.[55] Multiple ongoing studies are investigating ibrutinib in combination with various immunochemotherapy regimens, such as bendamustine and rituximab or ofatumumab.
GS-1101 is an isoform-selective inhibitor of phosphatidylinositol 3-kinase (PI3K) δ; it inhibits PI3K signaling and induces apoptosis of CLL cells in vitro.[56] A phase I trial using GS-1101 in relapsed CLL patients (n = 54) demonstrated that 84% achieved a ≥ 50% decrease in lymph node and spleen size. However, a > 50% increase in lymphocytosis (temporary in some-similar to the reaction seen with ibrutinib) was seen in 58%, and this resolved in only a subset of patients, thereby lowering the ORR by the IWCLL criteria.[17] The response rate seen in this study was independent of typical high-risk CLL features, notably the presence of del(17p13.1) (n = 19) and bulky adenopathy (n = 44). Median PFS had not been reached at the time of presentation but was > 11 months. Notably, del(17p13.1) patients had a shorter PFS in this study than did other groups. The compound was well tolerated, with limited toxicity reported.[57] Multiple combinations involving GS-1101 are currently in phase II trials, including combinations with rituximab, ofatumumab, and bendamustine and rituximab. Multiple other agents that target the BCR signaling pathway are currently under investigation in clinical trials in lymphoid malignancy, including fostamatinib, dasatinib (Sprycel), AVL-292, GDC-0941, and XL147.
CDK inhibitors
Another promising group of agents that have shown efficacy in the del(17p13.1) population are the CDK inhibitors, most notably flavopiridol and dinaciclib. CDKs are a family of proteins that control most of the steps in the cell cycle and also other functions, such as transcription (among the proteins in this family are CDK7, CDK8, and CDK9). The mechanism by which CDK inhibitors promote cell death in nonproliferating CLL cells has long been an enigma, although recent evidence with flavopiridol suggests endoplasmic reticulum stress is a major mechanism of action.[58] Clinical trials have indicated clinical efficacy for flavopiridol in the RR CLL population. A review of the major phase I[59] and phase II trials[60] involving flavopiridol noted that ORR and PFS were not significantly different between cytogenetic groups, with a 48% ORR and an estimated PFS of 10.1 months in the del(17p13.1) population.[61] Combinations of this drug with chemoimmunotherapy agents have been studied in the phase I setting, with successful results.[62,63] Dinaciclib is a small-molecule inhibitor of multiple CDKs that has been studied in a phase I trial in RR CLL. This trial demonstrated an ORR of 53% in patients with del(17p13.1) (n = 17).[64] Although the CDK inhibitors are efficacious, both flavopiridol and dinaciclib demonstrated increased risk of TLS in their early trials. However, this toxicity has been minimized by alteration of the dosing regimens and aggressive inpatient supportive care during the first two doses of the drug, when the tumor burden is at its highest. Neither of these drugs are currently commercially available.
BCL-2 antagonists
Another novel group of agents are the BCL-2 antagonists navitoclax (ABT-263) and ABT-199. Navitoclax binds to BCL-2, BCL-x1, and BCL-w, thereby halting suppression of BAX and BAK and allowing these proteins to oligomerize and trigger apoptosis of the tumor cells (see Figure 1). In a phase I trial in RR CLL, navitoclax has shown promise by reducing lymphocytosis by more than 50% in 19 of 21 patients (with baseline lymphocytosis). In patients treated with doses ≥ 110 mg/d (n = 26), 35% achieved PR and 33% maintained SD for > 6 months. Similar responses were shown in high-risk groups, including PR in 3 of 9 patients with del(17p13.1), with PFS not yet reached at time of publication. The major limiting side effect in this trial was dose-dependent thrombocytopenia, thought to be related to inhibition of BCL-x1.[65] Based on these promising data with navitoclax, another BCL-2 inhibitor, ABT-199, is currently under investigation in phase I trials. ABT-199 is an agent with more specific inhibition of BCL-2; it is hoped that it will be able to limit the thrombocytopenia toxicity while maintaining the clinical efficacy seen with navitoclax.
Conclusion
In summary, the population of CLL patients with del(17p13.1) or other defects in the p53 pathway has consistently demonstrated a poor response to therapy. The p53 defect impairs the CLL cell’s ability to respond to DNA damage inflicted by cytotoxic chemotherapy. Although progress has been made since the clinical significance of this abnormality was discovered, the currently available regimens are substandard, with poor response or unacceptable toxicity. Exciting new agents, which are designed to function in the absence of a functional p53 pathway, are showing much promise, as demonstrated in early clinical trial results. New therapies are clearly needed for the high-risk population of patients with del(17p13.1). Until such therapies are available, we would emphasize the importance of referring these individuals for clinical trials.
Financial Disclosure:Dr. Byrd has received funding for clinical support for trials from Pharmacyclics. Dr. Stephens has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
REFERENCES
1. Isobe M, Emanuel BS, Givol D, et al. Localization of gene for human p53 tumour antigen to band 17p13. Nature. 1986;320:84-5.
2. Zenz T, Habe S, Denzel T, et al. Detailed analysis of p53 pathway defects in fludarabine-refractory chronic lymphocytic leukemia (CLL): dissecting the contribution of 17p deletion, TP53 mutation, p53-p21 dysfunction, and miR34a in a prospective clinical trial. Blood. 2009;114:2589-97.
3. Dicker F, Herholz H, Schnittger S, et al. The detection of TP53 mutations in chronic lymphocytic leukemia independently predicts rapid disease progression and is highly correlated with a complex aberrant karyotype. Leukemia. 2009;23:117-24.
4. Oscier DG, Gardiner AC, Mould SJ, et al. Multivariate analysis of prognostic factors in CLL: clinical stage, IGVH gene mutational status, and loss or mutation of the p53 gene are independent prognostic factors. Blood. 2002;100:1177-84.
5. Wickremasinghe RG, Prentice AG, Steele AJ. p53 and Notch signaling in chronic lymphocytic leukemia: clues to identifying novel therapeutic strategies. Leukemia. 2011;25:1400-7.
6. Levine AJ. P53, the cellular gatekeeper for growth and division. Cell. 1997;88:323-31.
7. Badoux XC, Keating MJ, Wierda WG. What is the best frontline therapy for patients with CLL and 17p deletion? Curr Hematol Malig Rep. 2011;6:36-46.
8. Pietsch EC, Sykes SM, McMahon SB, Murphy ME. The p53 family and programmed cell death. Oncogene. 2008;27:6507-21.
9. Miyashita T, Krajewski S, Krajewska M, et al. Tumor suppressor p53 is a regulator of Bcl-2 and Bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799-1805.
10. Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910-6.
11. Stilgenbauer S, Zenz T, Winkler D, et al. German Chronic Lymphocytic Leukemia Study Group: Subcutaneous alemtuzumab in fludarabine-refractory chronic lymphocytic leukemia: clinical results and prognostic marker analyses from the CLL2H study of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2009;27:3994-4001.
12. Castro JE. Treatment of patients with chronic lymphocytic leukemia with 17p deletion: the saga continues. Leuk Lymphoma. 2012;53:179-80.
13. Hallek M, Fischer K, Fingerle-Rowson G, et al. International Group of Investigators, German Chronic Lymphocytic Leukaemia Study Group: Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;
376:1164-74.
14. Tam CS, Shanafelt TD, Wierda WG, et al. De novo deletion 17p13.1 chronic lymphocytic leukemia shows significant clinical heterogeneity: the M. D. Anderson and Mayo Clinic experience. Blood. 2009;114:957-64.
15. Oscier D, Wade R, Davis Z, et al. Chronic Lymphocytic Leukaemia Working Group, UK National Cancer Research Institute: Prognostic factors identified three risk groups in the LRF CLL4 trial, independent of treatment allocation. Haematologica. 2010;95:1705-12.
16. Rossi S, Shimizu M, Barbarotto E, et al. microRNA fingerprinting of CLL patients with chromosome 17p deletion identify a miR-21 score that stratifies early survival. Blood. 2010;116:945-52.
17. Hallek M, Cheson BD, Catovsky D, et al. International Workshop on Chronic Lymphocytic Leukemia: Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-56.
18. Badoux XC, Keating MJ, Wang X, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117:3016-24.
19. Fischer K, Cramer P, Stilgenbauer S, et al. The German CLL Study Group (GCLLSG). Bendamustine combined with rituximab (BR) in first-line therapy of advanced CLL: a multicenter phase II trial of the German CLL Study Group (GCLLSG). ASH Annual Meeting Abstracts. 2009;114:205.
20. Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29:3559-66.
21. Tsimberidou AM, Wierda WG, Plunkett W, et al. Phase I-II study of oxaliplatin, fludarabine, cytarabine, and rituximab combination therapy in patients with Richter’s syndrome or fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2008;26:196-203.
22. Thornton PD, Matutes E, Bosanquet AG, et al. High dose methylprednisolone can induce remissions in CLL patients with p53 abnormalities. Ann Hematol. 2003;82:759-65.
23. Bowen DA, Call TG, Jenkins GD, et al. Methylprednisolone-rituximab is an effective salvage therapy for patients with relapsed chronic lymphocytic leukemia including those with unfavorable cytogenetic features. Leuk Lymphoma. 2007;48:2412-7.
24. Castro JE, James DF, Sandoval-Sus JD, et al. Rituximab in combination with high-dose methylprednisolone for the treatment of chronic lymphocytic leukemia. Leukemia. 2009;23:1779-89.
25. Stilgenbauer S, Dohner H. Campath-1H-induced complete remission of chronic lymphocytic leukemia despite p53 gene mutation and resistance to chemotherapy. N Engl J Med. 2002;347:452-3.
26. Lozanski G, Heerema NA, Flinn IW, et al. Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood. 2004;103:3278-81.
27. Osuji NC, Del Giudice I, Matutes E, et al. The efficacy of alemtuzumab for refractory chronic lymphocytic leukemia in relation to cytogenetic abnormalities of p53. Haematologica. 2005;90:1435-6.
28. Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616-23.
29. Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554-61.
30. Pettitt AR, Matutes E, Oscier D. Alemtuzumab in combination with high-dose methylprednisolone is a logical, feasible and highly active therapeutic regimen in chronic lymphocytic leukaemia patients with p53 defects. Leukemia. 2006;20:1441-5.
31. Pettitt AR, Jackson R, Carruthers S, et al. Alemtuzumab in combination with methylprednisolone is a highly effective induction regimen for patients with chronic lymphocytic leukemia and deletion of TP53: final results of the national cancer research institute CLL206 trial. J Clin Oncol. 2012;30:1647-55.
32. Stilgenbauer S, Cymbalista F, Leblond V, et al. Subcutaneous alemtuzumab combined with oral dexamethasone, followed by alemtuzumab maintenance or allo-SCT In CLL with 17p- or refractory to fludarabine - interim analysis of the CLL2O trial of the GCLLSG and FCGCLL/MW. ASH Annual Meeting Abstracts. 2010;116:920.
33. Parikh SA, Keating MJ, O’Brien S, et al. Frontline chemoimmunotherapy with fludarabine, cyclophosphamide, alemtuzumab, and rituximab for high-risk chronic lymphocytic leukemia. Blood. 2011;118:2062-8.
34. Badoux XC, Keating MJ, Wang X, et al. Cyclophosphamide, fludarabine, alemtuzumab, and rituximab as salvage therapy for heavily pretreated patients with chronic lymphocytic leukemia. Blood. 2011;118:2085-93.
35. Wierda WG, Kipps TJ, Mayer J, et al. Hx-CD20-406 Study Investigators: Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;
28:1749-55.
36. Dreger P, Corradini P, Kimby E, et al. Chronic Leukemia Working Party of the EBMT: Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: the EBMT transplant consensus. Leukemia. 2007;21:12-17.
37. Jaglowski SM, Byrd JC. Novel therapies and their integration into allogeneic stem cell transplant for chronic lymphocytic leukemia. Biol Blood Marrow Transplant. 2012;18:S132-8.
38. Dreger P, Dohner H, Ritgen M, et al. German CLL Study Group: Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116:2438-47.
39. Schetelig J, van Biezen A, Brand R, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol. 2008;26:5094-100.
40. Howlader N, Noone AM, Krapcho M. SEER cancer statistics review, 1975-2009 (vintage 2009 populations), SEER stat fact sheet: chronic lymphocytic lymphoma. Based on November 2011 SEER data submission, posted to the SEER web site, April 2012; Accessed June 1, 2012.
41. James AF, Castro JE, Sandoval-Sus JD, et al. Rituximab and high-dose methylprednisolone for the initial treatment of chronic lymphocytic leukemia is associated with promising clinical activity and minimal hematologic toxicity. ASH Annual Meeting Abstracts. 2008;112:47.
42. Dredge K, Horsfall R, Robinson SP, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69:56-63.
43. Lapalombella R, Andritsos L, Liu Q, et al. Lenalidomide treatment promotes CD154 expression on CLL cells and enhances production of antibodies by normal B cells through a PI3-kinase-dependent pathway. Blood. 2010;115:2619-29.
44. Awan FT, Johnson AJ, Lapalombella R, et al. Thalidomide and lenalidomide as new therapeutics for the treatment of chronic lymphocytic leukemia. Leuk Lymphoma. 2010;51:27-38.
45. Ferrajoli A, Keating MJ, Wierda WG, et al. Lenalidomide is active in patients with relapsed/refractory chronic lymphocytic leukemia carrying unfavorable chromosomal abnormalities. ASH Annual Meeting Abstracts. 2007;110:Abstract 754.
46. Chanan-Khan A, Miller KC, Musial L, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24:5343-9.
47. Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291-7.
48. Wendtner CM, Hillmen P, Mahadevan D, et al. Final results of a multicenter phase 1 study of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:417-23.
49. Badoux XC, Keating MJ, Wen S, et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood. 2011;118:3489-98.
50. James DF, Brown JR, Werner L, et al. Lenalidomide and rituximab for the intitial treatment of patients with chronic lymphocytic leukemia (CLL). A multicenter study of the CLL Research Consortium. ASH Annual Meeting Abstracts. 2011;118:Abstract 291.
51. Badoux XC, Keating MJ, O’Brien S, et al. Final analysis of a phase 2 study of lenalidomide and rituximab in patients with relapsed or refractory chronic lymphocytic leukemia. ASH Annual Meeting Abstracts. 2011;118:Abstract 980.
52.Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012;120:1175-84.
53. Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182-9.
54. Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287-96.
55. O’Brien S, Furman R, Coutre S, et al. The Bruton’s tyrosine kinase inhibitor ibrutinib is highly active and tolerable in relapsed or refractory and treatment naive CC patients, updated results of a phase Ib/II study. EHA Meeting Abstracts. 2012:Abstract 0542.
56. Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078-88.
57. Coutre SE, Byrd JC, Furman RR, et al. Phase I study of CAL-101, an isoform-selective inhibitor of phosphatidylinositol 3-kinase P110d, in patients with previously treated chronic lymphocytic leukemia. ASCO Meeting Abstracts. 2011;29:6631.
58. Mahoney E, Lucas DM, Gupta SV, et al. ER stress and autophagy: new discoveries in the mechanism of action and drug resistance of the cyclin-dependent kinase inhibitor flavopiridol. Blood. 2012;120:1262-73.
59. Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399-404.
60. Lin TS, Ruppert AS, Johnson AJ, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012-8.
61. Woyach JA, Lozanski G, Ruppert AS, et al. Outcome of patients with relapsed or refractory chronic lymphocytic leukemia treated with flavopiridol: impact of genetic features. Leukemia. 2012;26:1442-4.
62. Lin TS, Blum KA, Fischer DB, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol. 2010;28:418-23.
63. Stephens DM, Maddocks K, Andritsos LA, et al. Phase I feasibility trial of cyclophosphamide, alvodocidib (flavopiridol) and rituximab (CAR) in patients with high-risk B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma. EHA Meeting Abstracts. 2012:Abstract 155.
64. Jones JA, Flynn JM, Andritsos LA, et al. Phase I study of the cyclin-dependent kinase (CDK) inhibitor dinaciclib (SCH727965 in patients with relapsed or refractory chronic lymphocytic leukemia. EHA Meeting Abstracts. 2012:Abstract 544.
65. Roberts AW, Seymour JF, Brown JR, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488-96.
66. Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol. 2006;24:437-43.
67. Woyach JA, Ruppert AS, Heerema NA, et al. Chemoimmunotherapy with fludarabine and rituximab produces extended overall survival and progression-free survival in chronic lymphocytic leukemia: long-term follow-up of CALGB study 9712. J Clin Oncol. 2011;29:1349-55.
68. Zent CS, Call TG, Shanafelt TD, et al. Early treatment of high-risk chronic lymphocytic leukemia with alemtuzumab and rituximab. Cancer. 2008;113:2110-8.