Targeted Therapy: Its Status and Promise in Selected Solid Tumors Part II
This second article in our two-part series on targeted therapies in solid tumors covers the emergence of targeted therapies for the treatment of two common malignancies: lung cancer and breast cancer.
This second article in our two-part series on targeted therapies in solid tumors covers the emergence of targeted therapies for the treatment of two common malignancies: lung cancer and breast cancer. In these two tumors, the identification of a promising target has led to successful preliminary applications, and eventually to further advances through drug development and the fine tuning of patient selection. As a result, the percentage of patients with breast or lung cancer who are benefiting from targeted agents has steadily increased, even if the majority are still treated with conventional cytotoxic regimens.
We also review the latest therapeutic strategies for colorectal and gynecologic cancers-because these offer an instructive contrast. The curative regimens that have been developed for these two tumors-even those in more advanced stages-have included combinations of surgery and/or radiation with chemotherapy. The Cancer Genome Atlas has revealed complexities in the biology of these tumors that underscore the fact that reliance on selective DNA-damaging agents such as platinums, antimetabolites, and antimitotic agents will continue for some time.
We conclude that the therapeutic progress that may arise from the study of molecular pathways will be due not only to the development of new targeted therapies, but also to a better understanding of older drugs developed empirically in the past. Taken together, these two types of advance illustrate the remarkable overall effect of modern cancer therapeutics’ focus on tumor biology and tumor immunology.
Introduction
In Part I, we covered selected solid tumors in which targeted therapies have had a major impact, transforming and often substituting for the therapeutic options available prior to the identification of suitable targets; these tumors included renal cell carcinoma, hepatocellular carcinoma, malignant melanoma, and a wide range of sarcomas. Our review was focused on answering four fundamental questions about the use of targeted therapy for each of these tumors:
1. What is the underlying tumor biology that is being targeted?
2. How “targeted” are the so-called “targeted drugs”?
3. Is the targeted therapy also suitable for immunomodulation and/or immunoconjugation?
4. In what way does the targeted therapy constitute a meaningful improvement over chemotherapy?
Here, in Part II, we continue this approach for disease areas in which 1) targeted therapies have had a major impact on special patient subsets (eg, in breast cancer and lung cancer) or 2) some degree of usefulness has been demonstrated for targeted therapies as their integration with established treatments has evolved (eg, in gynecologic and gastrointestinal malignancies). We do not specifically address prostate cancer therapeutics, an area in which major strides have led to improved survival and resulted in US Food and Drug Administration (FDA) approval of four new drugs in the past 2 years. These advances have come about through efforts to strengthen the inhibition of androgens and/or androgen receptor signaling-long-established targets[1,2]-by harnessing immunity against prostate-specific antigens,[3] and by introducing a taxane that overcomes resistance to docetaxel. Advances resulting from improved targeting will undoubtedly be reflected in better outcomes for patients diagnosed with this disease in 2012, thereby rendering prostate cancer another “high-impact area.” We chose not to cover the remarkable changes that are taking place in prostate cancer therapeutics because several of the new drugs have only recently received FDA approval and are just entering the clinical arena-as well as for the sake of brevity. Another area of impact not covered in this review is thyroid cancer. The landscape of thyroid cancer treatment is changing: once a patient’s disease has become refractory or not amenable to treatment with I131, clinical benefit has been established for certain drugs that inhibit receptor tyrosine kinases (RTKs) relevant to oncogenesis and/or angiogenic pathways in specific cellular subtypes, leading to FDA approval (Table 1). Thyroid cancer may soon be recognized as still another area on which the impact of targeted therapies is becoming sizable and is resulting in the modification of pathologic classifications according to oncogenetic changes.
TABLE 1
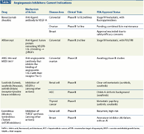
Angiogenesis Inhibitors: Current Indications
Areas Where Targeted Therapies Have Had an Impact on Special Tumor Subsets
The discovery of key molecular targets in some patients with cancers of the breast and lung opened up therapeutic opportunities in identifiable subsets in these diseases, eventually leading to the routine embracing of molecular profiling for treatment selection upon diagnosis. Demonstration that trastuzumab (Herceptin) added to chemotherapy in gastric cancer yielded benefits in patients with human epidermal growth factor receptor 2 (HER2) overexpression[4] has stimulated interest in routine testing for HER2 overexpression in this disease, and the discovery may herald a trend for use of such a strategy in settings beyond these common cancers.
Breast cancer
1. What is the underlying tumor biology that is being targeted? Decades after the discovery by Jensen et al[5] of estrogen receptors (ERs) in rodent endometrium, Perou et al[6] introduced a molecular classification-now widely embraced by clinicians-in which breast cancer biology was defined primarily by the presence or absence of hormone receptors. This took place after amplification of the oncogene HER2 joined the estrogen receptor as recognized powerful tumorigenetic mechanisms in breast cancer, providing further stimulus for the adoption of a classification based on genomics.[7] Successful targeting led to robust drug development and galvanized efforts to better define remaining subsets (usually identified within the “triple-negative” subset). The biology of this last broadly defined category is characterized by marked genetic instability, BRCA1 mutations, and activation of other angiogenic and epithelial growth factor receptor (EGFR) pathways.
Adjuvant and advanced-disease clinical trials are proceeding separately in these three categories: hormone receptor–positive, HER2-positive, and triple-negative. Trials do not yet separate luminal A and luminal B subtypes but are focusing on hormone resistance and concomitant blockade of downstream pathways that may account for the frequent failure of hormonal agents in patients with luminal B tumors. These hormone-dependent subsets are also characterized by delayed recurrences; this finding has stimulated research into the dormancy of stem cells, and into immune eradication of these tumor cells. Clinical successes achieved by targeting HER2 have led to further insight into signaling pathways within this family of receptors[8] and to the testing of small-molecule drugs as well as antibodies. Immunotherapy and immunoconjugates already play a major role in the treatment of HER2-overexpressed tumors. On the other hand, strategies against triple-negative tumors have included chemotherapy in combination with RTK inhibitors that are either highly selective or “promiscuous” (targeting a range of vascular endothelial growth factor receptors [VEGFRs] and EGFRs). Thus, although much remains to be defined in the tumor biology of breast cancer, the road map for integrating targeted therapies into our clinical trials is well on its way.
2. How “targeted” are the so-called “targeted drugs”? ER targeting has been ongoing since the discovery of ERs, starting with the early use of pharmacologic doses of hormones and ablative surgeries and the introduction of selective estrogen receptor modulators (SERMs) in the 1970s. The past two decades have witnessed the superior benefits conferred by third-generation aromatase inhibitors and ER down-regulators. The focus has now shifted to understanding downstream pathways and the reasons that ER inhibition proves to be inadequate. The double-blind randomized BOLERO-2 trial examined the role of the mammalian target of rapamycin (mTOR) inhibitor everolimus (Afinitor) (vs placebo) added to second-line treatment with an aromatase inhibitor in advanced breast cancer: it provided persuasive evidence of “cross-talk” with other key targets when targeting the ER alone has limited benefit.[9] However, it is HER2 that continues to be the quintessential target to be exploited for clear benefit, since as many as 25% of breast cancer patients have amplification of this growth factor receptor. Trastuzumab, an antibody to the extracellular domain of HER2, has dominated the treatment of patients with HER2-amplified tumors since it led conclusively to improved outcomes in combination with chemotherapy for advanced disease, and most strikingly as adjuvant therapy.[10] The RTK inhibitor lapatinib (Tykerb), which targets the catalytic domain of HER2, also attained an established role in the treatment of advanced breast cancer patients who had failed trastuzumab. Additional breakthroughs include the recent approval of pertuzumab (Perjeta) by the FDA; pertuzumab, an antibody that binds to a different extracellular domain of HER2, appears to act in tandem with trastuzumab to further inhibit proliferation or the development of resistance to pathway inhibition.[11] This encouraging area of research (discussed further below) represents an important example of the way in which key targets have the potential to bring about dramatic advances, including in areas beyond breast cancer.
3. Is the targeted therapy also suitable for immunomodulation and/or immunoconjugation? As noted, a second monoclonal antibody (pertuzumab) potentiates the action of trastuzumab (as seen, for example, in the CLEOPATRA trial[11]); it is possible that this effect is due in part to immune mechanisms. Also, the conjugation of a derivative of the potent mitotic inhibitor maytansine with trastuzumab-to form trastuzumab-DM1-has resulted in improved outcomes and clear advantages in the therapeutic index compared with a combination of docetaxel with trastuzumab. This is the first example of a successful immunoconjugate in an epithelial tumor (although the concept is well established in leukemias and lymphomas).[12] Immunoliposomes with trastuzumab projecting on the surface of a liposome containing pegylated liposomal doxorubicin represent another type of immunoconjugation-one that can potentially enhance the actions of chemotherapy on tumor cells with the target of interest.[13]
4. In what way does the targeted therapy constitute a meaningful improvement over chemotherapy? Breast and prostate cancers were the first examples of tumors in which the exploitation of molecular targets (ie, hormone receptors) was used to control the cancer. The adjuvant treatment of breast cancer with chemotherapy was the crown jewel of chemotherapy-related advances, but the successes seen with this approach have now paled in comparison to the effects of adjuvant trastuzumab (in combination with chemotherapy) when given to patients in whom HER2-overexpressing cancers have been diagnosed. This development has shifted the focus of research to “target-selected” clinical trials and a more extensive understanding of molecular pathways, including those mediated by hormone receptors.
Lung cancer
1. What is the underlying tumor biology that is being targeted? The study of the histological subtypes of lung cancer and their relationship to smoking coincided with the birth of chemotherapy and with awareness of this disease as a public health menace. By the 1970s, the National Cancer Institute had become involved in therapeutic research through its extramural and intramural programs.[14,15] Recognition of the biological features that help distinguish between small-cell carcinomas, squamous cell carcinomas, adenocarcinomas, and large-cell anaplastic carcinomas, as well as the less common neuroendocrine lung cancer subtypes, increased steadily over the next three decades. Currently, these distinctions are becoming reinvigorated through molecular profiling that has identified specific activating mutations in adenocarcinomas that are increasingly recognized and effectively targeted, although such studies are less developed in the other major subtypes. Lung cancer varies with ethnicity and smoking history, pointing to major genetic and environmental etiologic factors.
The main focus of targeted therapy in lung cancer has been EGFR. EGFR is an RTK that forms part of an RTK family that includes HER2 (the human epithelial receptor [HER] superfamily; Figure 1)-which, as described earlier, is a powerful target in breast cancer. Once activated, these tyrosine kinases drive both the RAS and the phosphatidylinositol 3 (PI3) kinase pathways, resulting in proliferation and suppression of apoptosis.[16] Both overexpression of EGFR and activating mutations in the kinase domain of the intracellular portion of the RTK have been demonstrated in responding lung adenocarcinomas; however, initial patient selection for clinical trials of EGFR-targeted drugs-as with trials of other systemic agents-did not discriminate among subtypes of non−small-cell lung cancer (NSCLC).[17] Once activating mutations were correlated with response, an intensive search for other changes in the kynome began. EML4-ALK is a fusion protein that is the result of an inversion in chromosome 2p that leads to the fusion of the echinoderm microtubule−associated protein-like 4 (EML4) gene and the anaplastic lymphoma kinase (ALK) gene.[18] The function of the fusion protein is under study; however, ALK itself is a target of interest in other malignancies, and it partners with other genes to create novel proteins implicated in tumorgenesis.[19] Approximately 4% to 5% of NSCLC may be driven by ALK fusion products.
TABLE 2
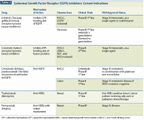
Epidermal Growth Factor Receptor (EGFR) Inhibitors: Current Indications
2. How “targeted” are the so-called “targeted drugs”? More than a decade ago, erlotinib (Tarceva) and gefitinib (Iressa) were the first small molecule tyrosine kinase inhibitors introduced clinically to selectively block EGFR signaling, and they showed some antitumor activity (Table 2). As noted earlier, their benefit was later related to the presence of activating mutations of EGFR. In fact, the Iressa Pan Asia Study (IPASS), which examined the efficacy of gefitinib vs carboplatin and paclitaxel in patients with metastatic pulmonary adenocarcinoma, demonstrated that gefitinib was significantly more effective than chemotherapy given as first-line therapy in patients with EGFR mutations[20]; on the other hand, in patients without EGFR mutations, chemotherapy was superior to gefitinib. The OPTIMAL study compared erlotinib to gemcitabine (Gemzar)/carboplatin in Chinese patients (with untreated EGFR-mutated NSCLC); it found a considerable progression-free survival advantage for erlotinib compared with chemotherapy.[21] Patients with KRAS mutations tend not to respond to such EGFR inhibition, presumably because KRAS is downstream of EGFR signaling.
Crizotinib (Xalkori) is a small-molecule inhibitor of ALK and c-MET tyrosine kinases that binds to the adenosine triphosphate (ATP) binding site of the ALK enzyme, thus preventing its ATP-mediated autophosphorylation. Crizotinib is highly selective for this family of tyrosine kinases and has been shown to be effective in the treatment of NSCLC patients with EML4-ALK fusion genes.[18,22]
3. Is the targeted therapy also suitable for immunomodulation and/or immunoconjugation? EGFR targeting has also been achieved with cetuximab (Erbitux), an antibody to EGFR, which has been shown to be of benefit in tumors that overexpress EGFR on the cell surface.[23] Immunoconjugation with cytotoxic elements has not yet been studied. Interestingly, ipilimumab (Yervoy; discussed in Part I of this review), the antibody to CTLA-4, has shown some promise in treating NSCLC. While the study of this unique antibody is still in early stages, preliminary data on this and other antibodies that target endogenous modulators of the immune system, such as Programmed Death (PD)-1 and its ligand PD-L1, suggest that we will soon need to consider immunomodulation in treatment plans for NSCLC.
4. In what way does the targeted therapy constitute a meaningful improvement over chemotherapy? Mutational analysis of NSCLCs is becoming nearly essential for patient management because of its prognostic and predictive value. As new mutations are discovered and subsequently targeted for treatment, growth in our armamentarium is foreseen against all cell types. Targeted therapy has not yet shown a meaningful benefit in the adjuvant setting, but it is likely that future therapeutic designs will incorporate these agents. Hopefully, the currently grave prognosis for most patients with advanced lung cancer and the high incidence of relapse in early-stage disease[24-26] will be altered by the results of targeted therapy trials. Alternatives that delay initiation of platinum doublet chemotherapy also carry with them a quality-of-life benefit for patients. Thus, targeted therapies are an impressive step forward in the treatment of NSCLC, and provide the basis for future meaningful steps.
Areas Where the Effective Integration of Targeted Therapies With Established Therapies Has Been Evolving
Steady progress has been made in the treatment of colorectal and gynecological cancers (as well as in the treatment of other cancers-such as bladder, brain, breast, gastroesophageal, and head and neck cancers) through multimodal strategies that have relied on surgery, radiation, and chemotherapy. In colorectal and ovarian cancers, anti-angiogenic agents have been the first targeted drugs to be successfully added to established combined-modality regimens, while other leads are being pursued based on emerging tumor biology.
Gastrointestinal cancers
This section focuses on the biology of metastatic colorectal cancers, where major strides have recently been made in the incorporation of targeted therapies.
1. What is the underlying tumor biology that is being targeted? Bert Vogelstein et al first articulated the sequential molecular changes that take place in colonic epithelium, starting from dysplasia and progressing through adenoma to carcinoma.[27] After colorectal cancer develops and eventually metastasizes, the tumor cells rely on angiogenesis, as described by Folkman[28] more than 5 decades ago. VEGFs that bind to VEGFRs are involved in new vessel formation via activation of downstream signaling and orchestration of endothelial cellular growth and function, as described by Hanahan and Folkman.[29] The most studied ligand in tumor angiogenesis is VEGF-A-a member of the VEGF family, which includes three other VEGF ligands. In colorectal cancers, VEGF-A is expressed in higher levels in distant metastases than in the primary tumor[30] and is an attractive target-one that is more significant when elevated levels of VEGF-A are accompanied by increased VEGFR expression. In fact, expression of VEGFR-1, the receptor for VEGF-A, increases with tumor grade and lymph node involvement.[31] VEGF-A also binds to VEGFR-2, a receptor that is upregulated in metastases. Several other factors currently being identified, which are involved in the growth and quality of blood vessels (eg, angiopoietins), will likely be the subject of future clinical study.
FIGURE 1
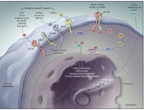
Activation of EGFR-Dependent Intercellular Signaling
The EGFR pathway constitutes another well-known target under investigation: 90% of metastatic colorectal cancers have EGFR overexpression, and this is associated with resistance to chemotherapy.[32,33] EGFRs belong to the previously mentioned superfamily of human epithelial receptors (HERs; see Figure 1) Like the VEGF family, the HER family also has multiple ligands, including EGF and transforming growth factor alpha (TGF-alpha). Several ligands can bind to EGFR (HER1), and once bound, EGFR dimerizes with any one of the HER family receptors, leading to downstream signal activation that results in cellular proliferation, metastatic spread, and neo-angiogenesis. Although EGFR inhibition became an early focus of targeted drug development in colorectal cancers as well as in other cancers, antitumor activity showed poor correlation with EGFR expression. The puzzle was partly solved when KRAS, one of the downstream signals of EGFR, was found to be mutated in up to 40% of metastatic colorectal cancers; constitutive activation of downstream RAS signaling was implicated in resistance to EGFR inhibition.[34] The downstream signals of EGFR and RAS include the PI3K and RAF kinase pathways, which are involved in tumor progression and survival. More recently, BRAF signaling (of importance in melanoma) has emerged as playing a role in driving proliferation in certain subsets of colorectal cancer patients even if KRAS is not mutated.[35] Our understanding of these pathways in colorectal cancer is expanding and will undoubtedly lead to new individualized approaches.
FIGURE 2
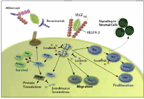
Angiogenic Pathways and Inhibitors Used in the Treatment of Colorectal Cancer and Other Tumors
2. How “targeted” are the so-called “targeted drugs”? Targeted therapy for colorectal cancer came of age in 2004, when bevacizumab (Avastin), a monoclonal antibody of VEGF-A, contributed to improvement of overall survival in combination with chemotherapy (fluorouracil [5-FU] and leucovorin) in metastatic colorectal cancer.[31] This landmark study gained FDA approval for bevacizumab and provided the foundation for the use of angiogenesis inhibitors in colorectal cancer. Aflibercept (Zaltrap) is a recombinant fusion protein comprised of VEGFR-1 and VEGFR-2 ligand-binding components fused with the constant region (Fc) of immunoglobulin G1 (IgG1) and inhibiting activation of these receptors (Figure 2). Aflibercept has recently demonstrated an overall survival advantage in patients with metastatic colorectal cancer that has progressed after first-line treatment (which could have included bevacizumab). The impact of these two targeted therapies-bevacizumab and aflibercept-has highlighted the role of angiogenesis in metastatic colorectal cancer and has renewed interest in other angiogenesis inhibitors, such as small molecules (see Table 1). As in other areas, major efforts are ongoing to reduce side effects and identify biomarkers associated with clinical benefit.
Cetuximab and panitumumab (Vectibix) are monoclonal antibodies that target EFGR. Much clinical experience has accumulated since the initial demonstration in colorectal cancer metastases of cetuximab’s reversal of clinical resistance to the topoisomerase I inhibitor irinotecan.[36] (Curiously, small molecules that narrowly target EGFR have not resulted in clinical benefit.) Both cetuximab and panitumumab were initially approved by the FDA for EGFR-overexpressing metastatic colorectal cancer, but such a basis for patient selection was questionable from the outset. A breakthrough in personalizing the clinical application of these drugs would not occur until the demonstration that the agents were ineffective in and possibly detrimental to patients whose tumors harbored KRAS mutations. Although no prospective studies to date have shown overall survival advantages, both agents extend progression-free survival in patients with KRAS wild-type tumors when combined with chemotherapy. Finally, regorafenib (Stivarga), an orally available small molecule inhibitor of VEGF-2 and -3, RAF kinases, and platelet-derived growth factor receptor (PDGFR), has demonstrated an impressive overall survival and progression-free survival advantage as a single agent (vs placebo) in patients who have progressed through standard therapies.[37] These findings provide further impetus for the continued study of these pathways in metastatic colorectal cancer.
3. Is the targeted therapy also suitable for immunomodulation and/or immunoconjugation? Efforts to develop vaccine therapies for metastatic colorectal cancer have been disappointing. A phase I study of autologous tumor cells modified to express interleukin-2 (IL-2) in colorectal cancer patients showed an increase of five times the normal level in the frequency of tumor cytotoxic T-cell precursors in a small number of patients following immunization.[38] Ipilimumab did not elicit responses in a phase I study that included three colorectal cancer patients,[39] and it is questionable whether CTLA-4 is an exploitable immune target in colorectal cancer.
4. In what way does the targeted therapy constitute a meaningful improvement over chemotherapy? Bevacizumab has helped redefine the concept of “synergy” of therapeutic interventions (already established in colorectal cancer after 5-FU + oxaliplatin was compared to merely additive 5-FU + irinotecan combinations). When added to FOLFOX (5-FU + folinic acid [leucovorin] + oxaliplatin [Eloxatin]), bevacizumab led to a significant prolongation in overall survival, suggesting that it made chemotherapy more effective. A similar improvement in survival has been achieved with aflibercept in the second-line setting. The other remarkable achievement of the application of targeted therapy concepts in colorectal cancer has been the definition of the role of KRAS pathways in the personalization of treatment: data mining in clinical trials demonstrated that molecular profiling that could identify when a tumor was KRAS wild-type predicted the usefulness of the EGFR antibodies, cetuximab and panitumumab. Thus, optimal treatment selection by oncologists is contributing not only to greater patient benefit but also to enhanced cost-effectiveness-a key element in the practice of medicine today.
Gynecologic cancers
1.What is the underlying tumor biology that is being targeted? Here we will discuss primarily high-grade serous cancers of extra-uterine Mllerian epithelial origin (the majority of ovarian, tubal, and primary peritoneal cancers). Endometrial cancers have been classified as type 1 (which result from atypical hyperplasia, and are generally ER-positive and well differentiated) and type 2 (which have a poorer prognosis and share many features with extra-uterine tumors). While type 1 tumors may be responsive to endocrine therapies, systemic treatment of type 2 tumors, especially uterine papillary serous cancers (UPSCs), consists of chemotherapy similar to that used in extra-uterine high-grade serous cancers.
The Cancer Genome Atlas (TCGA) has identified a vast number of genetic alterations among nearly 500 ovarian cancer specimens beyond stage I that displayed papillary serous histology at diagnosis.[40] Among the changes noted, somatic mutations in p53 were the most consistent findings, while somatic mutations in BRCA1, BRCA2, PI3Kinase, and PTEN were found in a small percentage (less than 10%) of the samples, with the occurrence of a mutation in any one of these genes excluding mutations in the others-a finding that tends to underscore the relevance of these mutations to oncogenesis. Amplification of c-MYC was found in more than 60% of the tumors included in TCGA, with KRAS showing a lesser extent of activation. The most common deletions involved PTEN, RB1, and NF1-and also PIK3R1. With regard to genomic and somatic mutations in BRCA1 and BRCA2 specifically, these were found in 20% of the high-grade serous ovarian cancers-and in another 8% one may find epigenetic silencing of BRCA1 via hypermethylation. The TCGA analysis has identified at least four gene expression subtypes, but the importance of these subtypes for personalizing treatment has not yet been determined. By contrast, platinum sensitivity is reflected by antitumor responses of as high as 80% on first exposure to these agents in stage III and IV tumors. Women treated with platinum-based regimens experience benefit that is sustained for a median of 18 months (and longer for those with minimal residual disease after surgical cytoreduction).[41] This somewhat unique sensitivity of ovarian cancer to cisplatin-which is exceeded only by the sensitivity of germ cell tumors-is now mostly attributed to alterations in homologous recombination (HR) DNA repair arising from defective BRCA function. This platinum sensitivity has been reproduced in genetically engineered mouse models of ovarian cancer[42] and is retained in the majority of cancer recurrences that occur 6 months or longer after treatment has been stopped (the definition of “platinum-sensitive disease”)-although retreatment with any number of platinum-containing doublets generally results in lower response rates and duration of response than were obtained on first remission. Defects in HR also confer increased sensitivity to other DNA-damaging drugs, such as pegylated liposomal doxorubicin (PLD)[43]; furthermore, these defects have been implicated in both in vitro[44,45] and clinical responsiveness[46] to inhibitors of base-excision DNA repair (poly[ADP-ribose] polymerase, or PARP)-ie, they lead to dual blockade and ensuing synthetic lethality). However, the platinum lesion on DNA, as shown in murine models, cannot be repaired without HR,[47,48] and the backbone of treatment of ovarian cancer remains platinum-based chemotherapy.[49] Although carboplatin achieves clinical results similar to those seen with cisplatin, on intraperitoneal (IP) administration, cisplatin may be more effective-consistent with a hypothesis that recurrences arise from less–cisplatin-sensitive cancer stem cells, while these cells’ progeny are still responsive to carboplatin or lower cisplatin doses.
Genomic changes in other histologic subtypes of ovarian cancer, such as endometrioid, clear-cell, and mucinous, are emerging but are not yet generally applied in clinical trials. Similarly, knowledge that folate receptor alpha is expressed in these epithelial tumors while being restricted to specialized normal tissues (such as the apical membrane of tubular cells, retinal pigment epithelium, and choroid plexus) has also been utilized in targeting.[50]
2. How “targeted” are the so-called “targeted drugs” In addition to PARP inhibitors (which remain experimental because of the paucity of phase III data on any one of the compounds under study), agents to which both ovarian and endometrial cancer have been responsive include the anti–VEGF-A antibody bevacizumab. Unlike its use in breast, colon, and lung cancers, in ovarian cancer bevacizumab appears to be useful as a single agent; in randomized trials it has contributed to prolongation of progression-free survival in first- and second-line platinum-sensitive relapses. In addition, the AURELIA trial, which tested PLD, paclitaxel, or topotecan alone vs with bevacizumab, demonstrated a significant contribution of this targeted agent to response rates and progression-free survival in the challenging treatment of platinum-resistant disease (the trial reported too few events to evaluate overall survival). Other anti-angiogenic targets have received less testing, but responsiveness to agents that target Tie-1 and Tie-2 (receptors for angiopoietins) or responsiveness to RTK inhibitors (eg, sorafenib [Nexavar]) has been noted; studies are ongoing with these drugs in combinations,[51,52] although toxicities are a concern.[53] Screening for HER2 overexpression as a target for trastuzumab proved futile in a Gynecologic Oncology Group study,[54] but this concept needs to be revisited in UPSC, since HER2 overexpression is more common in UPSC than in ovarian cancer.
Many ongoing trials are targeting the PI3K/AKT/mTOR pathway, but much remains to be learned about patient selection, molecular signatures that can predict for response, and whether pathway activation and mutations require different strategies for inhibition. Trials are also ongoing in endometrial cancer, where researchers hope to establish a link between resistance to endocrine therapy and activation of this pathway (perhaps analogous to what is seen in breast cancer). Striking preliminary results in ovarian cancer were reported in a study of cabozantinib, a potent MET inhibitor.[55] However, targeting individual pathways may not be appropriate for most ovarian cancers. In fact, given that the hallmark of serous ovarian cancers in The Cancer Genome Atlas is great variability in the genome from patient to patient, the discussant at the American Society of Clinical Oncology (ASCO) 2012 oral gynecologic cancer session that covered three targeted therapy trials made the suggestion that no phase III study of a targeted drug should be started without a full genome display as a criterion for entry.[56]
3. Is the targeted therapy also suitable for immunomodulation and/or immunoconjugation? The IP route of administrastion has some appeal for testing of immunomodulatory strategies. In fact, studies with IP IL-2 and lymphokine-activated killer (LAK) cells yielded some long-term survivors. In addition, reversal of platinum resistance has been postulated to occur with agents such as cyclosporine or the proteasome inhibitor bortezomib (Velcade). Attempts at delivering an adenovirus vector conferring wild-type p53 have been made in the past, but studies beyond phase I have not been completed. A sustained response to ipilimumab in one patient sparked interest in trials in ovarian cancer, but activity has not been reproduced.[57]
Conclusions
Over the past decade, drug targeting has, justifiably, frequently taken center stage in cancer therapeutics. This overview, limited to selected areas in the treatment of solid tumors, provides already ample evidence of the major impact of targeting on outcomes in certain cancers and in specific subsets of other cancers. These gains have led to the expectation that whole-genome studies will eventually result in the identification of key “druggable” targets for all cancers. However, in at least two of the most common solid tumors-those arising in the colon and in the gynecologic tract-major strides took place against a backdrop of chemotherapy, and the picture is now one of somewhat empirical “evolving integration” of new targeted drugs with established therapeutic modalities. In fact, in high-grade serous ovarian cancers, genetic abnormalities are so numerous and varied that replacing current treatments with targeted approaches is unlikely. Hence, in these areas, therapeutic advances in the near future are not expected to come from characterizing the genome and selecting from a menu of targeted drugs. Rather, improved selectivity in the treatment of ovarian cancer, with its innumerable somatic mutations, will likely emerge from understanding repair of chemotherapy-induced DNA damage, from consequences of modulating neo-angiogenesis, or from harnessing the immune system after major tumor cytoreduction. The challenge in the future is not only one of target identification-what years ago we dubbed “reaching for the magic bullet summit” (ie, rational therapeutics in drug development). In addition, empiricism emanating from the study of biological systems (including clinical trials) is needed to explore which of a vast number of emerging drug products should be integrated into current therapies, and how this should be done.[58] These cautionary comments should not preclude continuing efforts to couple tumor biology with a search for molecular targets as the key to future advances. Hopefully, in the midst of the development of targeted therapies, this brief overview provides a perspective on how therapeutic progress against a range of solid tumors is being achieved.
Financial Disclosure: Dr. Muggia has served on safety data monitoring committees for Bayer, Lilly, Pfizer, and Roche. Dr. Joseph and Dr. Wu have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
REFERENCES
1. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005.
2. Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437-46.
3. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-22.
4. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-97.
5. Jensen EV, Desombre ER, Kawashima T, et al. Estrogen-binding substances of target tissue. Science. 1967;158:529-30.
6. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747-52.
7. Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177-82.
8. Rexer BN, Arteaga CL. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications. Crit Rev Oncog. 2012;17:1-16.
9. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520-9.
10. Burstein HJ. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005;353:1652-4.
11. Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109-19.
12. Teicher BA, Chari RV. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011;17:6389-97.
13. Park JW, Hong K, Kirpotin DB, et al. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8:1172-81.
14. Hansen HH, Muggia FM, Andrews R, Selawry OS. Intensive combined chemotherapy and radiotherapy in patients with nonresectable bronchogenic carcinoma. Cancer. 1972;30:315-24.
15. Minna JD, Bunn PA, Jr., Carney DN, et al. Experience of the National Cancer Institute (USA) in the treatment and biology of small cell lung cancer. Bull Cancer. 1982;69:83-93.
16. Coate LE, John T, Tsao MS, Shepherd FA. Molecular predictive and prognostic markers in non-small-cell lung cancer. Lancet Oncol. 2009;10:1001-10.
17. Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non–small-cell lung cancer (the IDEAL 1 trial). J Clin Oncol. 2003;21:2237-46.
18. Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693-703.
19. Johnson JL, Pillai S, Chellappan SP. Genetic and biochemical alterations in non-small cell lung cancer. Biochem Res Int. 2012;2012:940405.
20. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-57.
21. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735-42.
22. Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004-12.
23. Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525-31.
24. Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355:479-85.
25. Nesbitt JC, Putnam JB, Jr., Walsh GL, et al. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg. 1995;60:466-72.
26. Potti A, Mukherjee S, Petersen R, et al. A genomic strategy to refine prognosis in early-stage non-small-cell lung cancer. N Engl J Med. 2006;355:570-80.
27. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-67.
28. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-6.
29. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-64.
30. Barozzi C, Ravaioli M, D'Errico A, et al. Relevance of biologic markers in colorectal carcinoma: a comparative study of a broad panel. Cancer. 2002;94:647-57.
31. Rmali KA, Puntis MC, Jiang WG. Tumour-associated angiogenesis in human colorectal cancer. Colorectal Dis. 2007;9:3-14.
32. Arteaga CL. The epidermal growth factor receptor: from mutant oncogene in nonhuman cancers to therapeutic target in human neoplasia. J Clin Oncol. 2001;19:32S-40S.
33. Saif MW, Chu E. Biology of colorectal cancer. Cancer J. 2010;16:196-201.
34. Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-17.
35. Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;
29:2011-9.
36. Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-45.
37. Grothy A. Regorafenib in metastatic colorectal cancer. Clin Adv Hematol Oncol. 2012;10:324-5.
38. Sobol RE, Shawler DL, Carson C, et al. Interleukin 2 gene therapy of colorectal carcinoma with autologous irradiated tumor cells and genetically engineered fibroblasts: a Phase I study. Clin Cancer Res. 1999;5:2359-65.
39. O'Mahony D, Morris JC, Quinn C, et al. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin Cancer Res. 2007;13:958-64.
40. Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609-15.
41. National Cancer Institute. Ovarian epithelial cancer treatment (PDQ). Available from: http://www.cancer.gov/cancertopics/pdq/treatment/ovarianepithelial/HealthProfessional/page5. Accessed October 25, 2012.
42. Dinulescu DM, Ince TA, Quade BJ, et al. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63-70.
43. Safra T, Borgato L, Nicoletto MO, et al. BRCA mutation status and determinant of outcome in women with recurrent epithelial ovarian cancer treated with pegylated liposomal doxorubicin. Mol Cancer Ther. 2011;10:2000-7.
44. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917-21.
45. Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913-7.
46. Banerjee S, Kaye S. PARP inhibitors in BRCA gene-mutated ovarian cancer and beyond. Curr Oncol Rep. 2011;13:442-9.
47. Fasano J, Muggia F. Breast cancer arising in a BRCA-mutated background: therapeutic implications from an animal model and drug development. Ann Oncol. 2009;20:609-14.
48. Borst P, Rottenberg S, Jonkers J. How do real tumors become resistant to cisplatin? Cell Cycle. 2008;7:1353-9.
49. Muggia F. Platinum compounds 30 years after the introduction of cisplatin: implications for the treatment of ovarian cancer. Gynecol Oncol. 2009;112:275-81.
50. Visentin M, Zhao R, Goldman ID. The antifolates. Hematol Oncol Clin North Am. 2012;26:629-48, ix.
51. Lu JF, Rasmussen E, Karlan BY, et al. Exposure-response relationship of AMG 386 in combination with weekly paclitaxel in recurrent ovarian cancer and its implication for dose selection. Cancer Chemother Pharmacol. 2012;69:1135-44.
52. Lee JM, Sarosy GA, Annunziata CM, et al. Combination therapy: intermittent sorafenib with bevacizumab yields activity and decreased toxicity. Br J Cancer. 2010;102:495-9.
53. Cannistra SA. Challenges and pitfalls of combining targeted agents in phase I studies. J Clin Oncol. 2008;
26:3665-7.
54. Bookman MA, Darcy KM, Clarke-Pearson D, et al. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol. 2003; 21:283-90.
55. Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298-308.
56. Dizon DS. Redesigning studies: pushing the envelope. Available from: http://connection.asco.org/Commentary/Article/ID/3232/Redesigning-Studies-Pushing-the-Envelope.aspx. Accessed October 31, 2012.
58. Hodi FS, Butler M, Oble Da, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte–associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005-10.
59. Muggia FM, Rozencweig M, Editors. Evaluation of cancer treatment. The Hague: Martinus Neijoff; 1982.
60. Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160-1174.