The Cost of Cancer Care: Part II
In Part II, I focus on ideas and specific programs that may slow the growth of spending while, it is hoped, minimizing the impact on what we all want: sustainable access to high-quality therapy and continued innovation. Finally, I will consider another fundamental question: Is current spending worth it?
The rising cost of cancer treatment competes with the availability of effective therapy as a limiting factor in our war on cancer. Specific programs are being developed that have the potential to slow the growth in spending on oncology care. The Affordable Care Act includes provisions for containing healthcare costs, such as accountable care organizations and the Independent Payment Advisory Board. Within oncology, specific programs have emerged, including clinical pathways, episode-of-care based payment arrangements, and the oncology medical home. All models of cost containment have strengths and weaknesses. Outside of the United States, explicit rationing exists through national health technology assessment organizations. Excessive demands on physicians to limit spending at the bedside could potentially create conflicts with their professional responsibility to patients. While spending for cancer care in the US is high, its “worth” is ultimately a societal decision. Recent economic modeling suggests that we may be achieving value for the money we spend.
Oncology is reaping the benefits of accelerating scientific progress. Accumulating insights into the fundamental drivers of carcinogenesis at the cellular level are leading to the development of much more effective, less-toxic therapies. The timing of this phase of advance coincides with rising economic constraints within our field-and in all of medicine. Indeed, this new era of more-effective cancer therapeutics may be inherently more expensive as patients live longer on active treatment. The cost of treatment now competes with the availability of effective therapy as a limiting factor in our war on cancer.
In Part I of this article on the cost of cancer care, I examined what appear to be the fundamental drivers of the oncology cost-curve and the impact that cost is having on patients. In Part II, I focus on ideas and specific programs that may slow the growth of spending while, it is hoped, minimizing the impact on what we all want: sustainable access to high-quality therapy and continued innovation. Finally, I will consider another fundamental question: Is current spending worth it?
TABLE
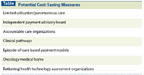
Potential Cost-Saving Measures
What Can We Do to Lower Costs?
A possible cultural shift may be emerging in medicine. From the American College of Physicians' proposal of parsimonious care to the American Board of Internal Medicine's Choosing Wisely Campaign, an explicit endorsement of limiting spending while providing care is being proffered.[1,2] It may be hoped that by creating an ethic of waste avoidance, we can delay or minimize more difficult decisions such as outright rationing of care.[3] Given limited resources, nonbeneficial spending on any given patient detracts from resources that could be used more effectively for another. (See Table for potential cost-saving measures.)
What efforts to limit spending are either in place or on the way?
Private payers often achieve cost control through “blunt instruments” that sometimes poorly serve their beneficiaries. “Tools of the trade” include prior authorization, compendia listing and guideline requirements, quantity limits for oral drugs, patient cost-sharing, and mandated third-party disease management. Without question, employers are urging insurers to reduce costs. In my experience, patients perceive that they have less recourse with their employee benefit managers when they are being poorly served by their insurance plan.
The Affordable Care Act includes flawed elements designed to slow the growth in health spending. The Independent Payment Advisory Board empowers a panel to limit payments to providers. Since reducing benefits to Medicare beneficiaries by the panel is proscribed, this process may result in lowering reimbursement for services below the cost of actually providing the services themselves. The Components of Care Survey, conducted by the Community Oncology Alliance and Avalere Health, concluded that payments for infusion services by Medicare covered only 57% of the cost to provide the care in 2008.[4] Accountable care organizations encourage cost control through gain-sharing arrangements in which providers and Medicare share in savings accrued relative to a benchmark. However, no quality measures exist within this model to ensure that cancer patients under active treatment are not denied appropriate care. The only two quality measures related to oncology address mammography and colorectal cancer screening.
Cancer patients under active treatment comprise 1% of a payer’s patients but as much as 10% of costs.[5] This leads to two questions: What novel cost-control initiatives in oncology have either been proposed or are being actively piloted? What are the strengths and limitations of these initiatives?
Clinical Pathways
Clinical pathways are specific, evidence-based tools designed to determine specific regimen use for a given stage of disease. While the level of detail within pathways can vary, they are more prescriptive than guidelines.[6] Savings can be achieved by choosing less-expensive but equally efficacious treatment protocols. An appropriate target for compliance, such as 70% to 85%, must be determined. Payers generally desire a mechanism for internal validation of pathway compliance.
Pathways are being implemented in diverse geographic regions and practice models. A statewide initiative in Michigan, for example, includes Blue Cross Blue Shield, physicians, and an oncology benefit management company.[7] Care pathways were first developed for breast, colon, and lung cancer, and later expanded to additional diseases. The pathway program included enhanced reimbursement: an upfront participation payment, increased payment for generics, and increased payment for evaluation and management codes.
The US Oncology Network has incorporated pathways into their electronic medical record system. US Oncology has compared cost-of-treatment and outcomes for non–small-cell lung cancer patients treated both on and off pathway. Outpatient costs were 35% lower for patients treated on pathway, and no difference in survival was noted. Savings appeared to be achieved by the use of less-expensive drugs and decreased use of therapy overall. [8]
Episode-Based Payments
Care for oncology patients, particularly in the adjuvant setting, is often given over predictable time frames. Instead of paying for each element of care separately, episode-of-care payments either can either pay a flat fee per unit of time or a flat fee for a defined care plan. The availability of accepted guidelines in oncology facilitates this payment approach.
Bach et al proposed such a model for metastatic lung cancer.[9] In this model, oncologists would receive a monthly payment derived from the average cost of caring for all patients with metastatic lung cancer. This payment would bundle the costs of chemotherapy, supportive care medications, and administration. Medicare payments would then be adjusted over time based on claims submitted during prior episodes. Physicians would have to demonstrate that treatment conformed to an accepted standard of care. The intent of the program would be to achieve savings by making physicians discretionary purchasers based on price. The downstream effect would also pressure pharmaceutical manufacturers to adjust drug prices downward in order to be economical within the structure of the payment model.
Even in theory, this model has several flaws. It is likely that many more flaws would be apparent with more widespread application of this model to other disease types. The proposed model was based only on the use of platinum doublets. Bevacizumab (Avastin), an expensive agent with demonstrated survival benefit and an option within current guidelines, was not included in the analysis. Compendia-listed therapies cannot simply be left out of the equation. Since the publication of this proposal, the use of targeted oral therapies based on tumor mutational status, and the broader selection of chemotherapy based on tumor histology, have continued to evolve. It would appear unlikely that the savings achieved through temporal payment adjustments can keep pace with the introduction of new applications of scientific advances. Finally, the model places additional financial risk with providers in order to indirectly pressure pharmaceutical pricing downwards. Oncology clinics are already closing at alarming rates and are poorly positioned to act as an indirect price-control mechanism for drug cost.[10]
United Healthcare is currently piloting a distinct version of episode-of-care payments in partnership with community practices.[11] The pilot involves the treatment of breast, lung, and colon cancers in both the adjuvant and metastatic setting.
In the adjuvant setting, episode-of-care payments are separated according to disease, stage, and relevant biologic markers, such hormone receptor status and HER2/neu (human epidermal growth factor receptor 2) for breast cancer. Practices then selected one treatment regimen for each adjuvant group and were required to use this regimen at least 85% of the time. When important therapies emerge as new standards of care, changes are made through consensus between the practices and United Healthcare. The operating margin for chemotherapy, supportive care drugs, and ancillary supplies is converted into an episode-of-care payment. In the adjuvant setting, this payment also included independent fees for chemotherapy management and hospitalization services. Drugs are reimbursed at average sales price figures. Separate payments are made for evaluation and management services and chemotherapy administration. Hospital service fees are included in the episode-of-care payment. Since no additional physician payments occur for hospitalized patients, physicians are incentivized to manage patients on an outpatient basis when medically appropriate.
In the metastatic setting, the episode-of-care payment is based on the national average of chemotherapy drug margin combined with payment for chemotherapy treatment planning and management of hospitalizations. The episode-of-care payment is made every 4 months, independent of whether the patient is under active treatment or enrolled in hospice services. Physician work for the palliative and hospice care is covered under this arrangement.
The results? Thus far, practices seem satisfied with this model despite increased administrative work. Involving physicians in the creation of both the program and the selection of regimens for treatment of their patients appears to be a key driver of success. Payments are better structured to cover the cognitive services of medical oncologists. These cognitive services form a key pillar of the value that oncologists deliver to patients. United Healthcare achieves improved predictability of costs and better alignment of incentives.
The Oncology Medical Home
The medical home model of oncology care is another critical opportunity in the evolving delivery of oncology care, to both ensure quality and reduce cost.[12] The model emphasizes improved care coordination, recognizing that fragmented care acts as an important cost driver in oncology. This model began with the efforts of Dr. John Sprandio with Consultants in Medical Oncology and Hematology, the first oncology practice to achieve level III recognition from the National Committee for Quality Assurance.
The model employs several elements, with its key strength being its synthesis of multiple separate but important efforts in oncology: care coordination, open access, quality measurement, guideline adherence, and cost savings by preventing emergency department (ED) visits and hospitalization. Patient performance status is a key metric for decision-making, including eligibility for chemotherapy administration. This helps to ensure that patients are appropriate for active treatment vs palliative care. Dr. Sprandio’s practice has achieved reductions in ED visits per chemotherapy patient by 68% and hospitalizations per chemotherapy patient by 51%.[13] These are meaningful accomplishments, since the cost of hospitalization may equal or exceed spending on oncology drugs.
This model has now further evolved into a unique multistakeholder effort which includes practicing physicians, private payers, professional societies, patient advocates, and others. The objectives of this multistakeholder effort include defining and implementing quality metrics. Identifying opportunities for responsible cost savings that do not threaten patient care quality or practice viability is a critical goal.
Rationing
Many potential cost-containment plans can be implemented. These include encouraging a cultural shift among healthcare providers to be more mindful in their utilization of resources. Programs such as those previously discussed have the potential to reduce cost without sacrificing quality or access. If these efforts fail or are insufficient, what additional measures may be required?
Rationing is a concept that many people in the United States abhor. For the purposes of this discussion, I will consider rationing as the withholding of care known to be clearly beneficial rather than care that is considered low value or of uncertain benefit. The very idea itself has become a political device for attacking opponents in the healthcare arena. Whereas the US system of healthcare may include some inherent elements of rationing, such as access according to insurance status, overt rationing remains distasteful to most. Drug approval by the US Food and Drug Administration (FDA) does not consider cost. Medicare pays for all treatment according to approved labeling and compendia listing.
Other developed Western countries include overt rationing in their healthcare systems.[14] Care of cancer patients often receives special consideration but does not remain immune from the process. The cost-effectiveness of oncology drugs receives scrutiny through various national health technology assessment organizations. Canada operates the pan-Canadian Oncology Drug Review. Australia has created the Pharmaceutical Benefits Advisory Committee.
The agency best known for cost-effectiveness assessment is the United Kingdom’s National Institute of Health and Clinical Excellence, familiar to most by its Orwellian acronym "NICE." The agency uses a cost-effectiveness threshold of approximately $50,000 US per quality-adjusted life-year. In the past, it has approved cancer therapies such as trastuzumab (Herceptin) for HER2-positive advanced breast cancer and imatinib (Gleevec) for chronic myeloid leukemia, despite the fact that the cost of these drugs exceeds this threshold. Many other oncology therapeutics have been rejected in the past few years. Public reprisals from some of these decisions prompted the agency to modify standards to include consideration of shorter life expectancy, survival benefit, and lack of alternative treatments with comparable efficacy. NICE now explicitly grants special consideration to oncology drugs.
The NICE model would certainly struggle within the context of the US political system. Despite this, we should recognize that NICE represents a prospectively agreed upon, transparent process. If rationing of care is going to ever exist in the US, better for it to be visible rather than disguised within some opaque bureaucratic operation. It is also important to remember that drug costs represent less than a quarter of the cost of oncology care in the US. Focusing on this alone will be insufficient to control cost and may endanger innovation during an era of critical scientific advance.
Can We Count on Physicians to Limit Spending at the Bedside?
Any system that uses a broad brush to define both the value of treatment and subsequent coverage is almost certain to lead to errors. These errors can easily occur in oncology due to heterogeneous patient populations and frequently unique patient situations. Since physicians best understand the specific situation of individual patients, they best understand their particular individual needs. They may be well positioned to balance the expected benefit of treatment vs the cost.
But at what point does a physician’s consideration of societal resources conflict with the professional responsibility to deliver the best possible care for his or her patient? Is this tension irreconcilable? It may be that bedside decisions regarding resource allocation fail because individual physicians judge the cost-effectiveness of treatment differently. They may also fail because physicians do not uniformly include cost-effectiveness considerations in their decision-making. Patients should not be subjected to variable degrees of access to treatment based on whom their physician happens to be.
Survey data demonstrate that most, but not all, oncologists believe that every patient should have access to effective cancer treatment irrespective of the cost of therapy.[15] Other survey data reveal wide discrepancies in what oncologists consider to be “good value for money.”[16] Most oncologists favor prescribing effective therapy even if they believe the therapy does not represent “good value.”[15]
In a sense, this is understandable. Oncologists practice in a complex environment in which many of the key structural and operational elements of healthcare are beyond their control. Oncologists have no authority over the pricing of therapies. Though they may consider the cost of a particular therapy to be excessive, they may feel less responsibility for the cost implications of care as long as it benefits patients. If sipuleucel-T (Provenge) happens to cost $93,000 for a course of therapy to deliver an expected 4-month median survival benefit, the high cost may not prompt them to withhold therapy as long as the patient is an appropriate candidate.
Is Current Spending on Oncology Care Worth It?
We are appropriately concerned about the rising cost of cancer care. But if we consciously decide to spend less on oncology, would we be giving up something that we actually want? Is the current 0.8% of gross domestic product spent on cancer care too much?[17]
When comparing the US health system with the healthcare systems of other countries, it is a common assertion that we spend far more for health outcomes that are equal to or even worse than those of countries that spend less. Recent statistics from the Organisation for Economic Co-operation and Development (OECD) glaringly illustrate this point.[18] Life expectancy in the US is below the average life expectancy in other developed nations, despite per capita spending on healthcare that is more than double the average spending of these other nations. Lifestyle and health behaviors also affect life expectancy, leaving in question the direct impact of the various health systems on longevity. Nevertheless, overall we appear to be doing something wrong.
How are we doing with cancer care specifically?
Screening rates for cervical cancer in the US are the highest among OECD nations. However, the 5-year mortality rate for cervical cancer is below average. The US ranks third for screening mammography. The breast cancer 5-year relative survival rate is the highest in the world. Survival of colon cancer in the US is fourth among the 34 OECD member countries.[17] While international comparison of cancer survival is complex and typically relies on registry data, these figures are encouraging.
A recent analysis, comparing outcomes in the US and Europe, examined the apparently superior US outcomes relative to the increased cost.[19] Survival differences were determined according to registry data. A year of additional life was valued at $150,000-a reasonable metric in health economics. Cost of cancer care across nations was determined by multiplying the total cost of care in each country with the proportion of healthcare spending devoted to oncology. When applying these parameters, cancer care spending in the US is, in fact, worth it. Survival gains achieved over the last several years appear to be worth more than the relative growth of the cost of cancer care.
Without question, economic modeling is complex and requires multiple assumptions, and the data used in the modeling may be imperfect. Nevertheless, as we aim to curtail spending on cancer care, we should be cognizant that perhaps our efforts are worth it. Practicing oncologists are acutely aware of how greatly patients value access to the best available therapy, and the demand for quality oncology care and therapies by those who need it is extremely high. Individual consumers may prefer to spend their money on iPads, movie tickets, or other more enjoyable items and activities rather than health insurance. This is completely understandable. We enjoy spending on what we want far more than on what we need. But what we need is certainly important to us as well, particularly when battling complex, life-threatening illness.
Conclusions
Historically, successful treatment for cancer was limited primarily by lack of effective therapeutics. Now, with the emergence of novel and highly effective therapy, the cost of care has become an increasingly constraining factor. The drivers of the oncology cost curve are both complex and dynamic. It may be that the recent cost of oncology care comprises the same proportion of national health spending that it did two decades prior. Nevertheless, the present rate of increased spending is considered by many to be unsustainable. Existing mechanisms of cost control, such as patient cost-sharing, often result in personal bankruptcy or lack of access to therapy.
The scientific community continues to produce enormous innovation for the treatment of cancer. Now, innovation is needed in the process of care delivery. Using resources effectively, avoiding expensive hospitalizations, and improving operational efficiency will likely all be a part of this solution. In my view, the greatest prospect for success is to facilitate solutions developed by practicing physicians who best understand the delivery of care.
Financial Disclosure: The author has no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
References
1. Snyder L, for the American College of Physicians Ethics, Professionalism, and Human Rights Committee. American College of Physicians Ethics Manual. Ann Intern Med. 2012;156:73-104.
2. Schnipper L, Smith T, Raghavan D, et al. American Society of Clinical Oncology indentifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;30:1715-24.
3. Brody H. From an ethics of rationing to an ethics of waste avoidance. N Engl J Med. 2012;366;21:1949-51.
4. Avalere Research: Providing high quality care in community oncology practices: an assessment of infusion services and their associated costs. Feb 2010. Available from: http://www.avalerehealth.net/research/ereport.php?rid=1044. Accessed Oct 2, 2012.
5. Milliman, Inc, Fitch K, Pyenson B. Cancer patients receiving chemotherapy: opportunities for better management. Milliman Client Report. March 30, 2010. Available from: http://publications.milliman.com/research/health-rr/pdfs/cancer-patients-receiving-chemotherapy.pdf. Accessed Oct 2, 2012.
6. Gesme DH, Wiseman M. Strategic use of clinical pathways. J Oncol Pract. 2011;7:54-6.
7. Feinberg B, Lang J, Grzegorczyk J, et al. Implementation of cancer clinical care pathways: a successful model of collaboration between payers and providers. J Oncol Pract. 2012;8(3 Suppl):e38s-43s.
8. Neubauer M, Hoverman J, Kolodziej M, et al. Cost effectiveness of evidence-based treatment guidelines for the treatment of non-small-cell lung cancer in the community setting. J Oncol Pract. 2010;6:12-18.
9. Bach P, Mirkin J, Luke J. Episode-based payment for cancer care: a proposed pilot for medicare. Health Aff (Millwood). 2011;30:500-9.
10. Community Oncology Alliance. Community oncology practice impact report. April 4, 2012. Available from http://www.communityoncology.org/pdfs/
community-oncology-practice-impact-report.pdf. Accessed Oct 2, 2012.
11. Gould B. The “episode of care” payment model: one practice’s experience. Oncology (Williston Park). 2011;25:1310,1317.
12. Eagle D, Sprandio J. A care model for the future: the oncology medical home. Oncology (Williston Park). 2011;25:571,575-6.
13. Sprandio J. Oncology patient-centered medical home. J Oncol Pract. 2012;8(3 Suppl):47s-9s.
14. Neuman P, Bliss S, Chambers J. Therapies for advanced cancer pose a special challenge for health technology assessment organizations in many countries. Health Aff (Millwood). 2012;31:700-8.
15. Nadler E, Eckert B, Neumann P. Do oncologists believe new cancer drugs offer good value? The Oncologist. 2006;11:90-5.
16. Ubel P, Berry S, Nadler E, et al. In a survey, marked inconsistency in how oncologists judged value of high-cost cancer drugs in relation to gains in survival. Health Aff (Millwood). 2012;31:709-17.
17. Pauly M. Is high and growing spending on cancer treatment and prevention harmful to the United States economy? J Clin Oncol. 2007;25:171-4.
18. Organisation for Economic Co-operation. Health at a glance 2011: OECD indicators. Available from: http://www.oecd.org/health/healthpoliciesanddata/49105858.pdf. Accessed October 3, 2012.
19. Philipson T, Eber M, Lakdawalla D, et al. An analysis of whether higher health care spending in the United States versus Europe is “worth it” in the case of cancer. Health Aff (Millwood). 2012;31:667-74.