Classic phase II trials: Unable to meet the challenges in oncology, or an integral aspect of drug development?
Shortages in funding, manpower, and willing patients have created the proverbial perfect storm in the current clinical trial system. The fact that traditional clinical trial endpoints in assessing novel agents are being reconsidered only puts more pressure on an already strained system. As the cancer research community navigates the troubled waters of clinical trials, one question is: Should traditional phase II trials be phased out?
Shortages in funding, manpower, and willing patients have created the proverbial perfect storm in the current clinical trial system. The fact that traditional clinical trial endpoints in assessing novel agents are being reconsidered only puts more pressure on an already strained system. As the cancer research community navigates the troubled waters of clinical trials, one question is: Should traditional phase II trials be phased out? Richard S. Kaplan, MD, spoke for modifying traditional phase II clinical trials while Alberto Sobrero, MD, defended them during a debate at ECCO/ESMO 2009 in Berlin. Dr. Kaplan, an associate director of the National Cancer Research Network in the UK, argued that traditional phase II clinical trials suffer from unreliable outcomes among other problems. But Dr. Sobrero, director of medical oncology at Ospedale San Martino in Genoa, Italy, countered that phase II trials are worth keeping as long as certain caveats are taken into consideration.

Limited benefits with current phase II set-up
“Traditional phase II trial design is a legacy of the formative years of oncology, where needs and assumptions were different,” Dr. Kaplan said. “Traditional phase II studies need to change to address the fundamental challenges for oncology trials, including more rapid development and testing of new agents, shortening time to patient access, reliable assessment of drug activity, and identification of responding cohorts.”
Dr. Kaplan pointed out that by analyzing the “track record” of phase III trials, the problem with traditional phase II trials becomes clear. Conventional phase II trials are adept at ruling out inactive or toxic monotherapies, he explained. But traditional phase II trials are not good at screening out unpromising combinations and identifying treatments that are more likely to prove effective in a phase III trial.
“Only about 30% of oncology drugs in phase III trials are brought to market, less than half the rate for anti-infectives or cardiovascular agents. And we carry out at least three major phase III trials for every one that is positive,” he said. Other weaknesses of traditional phase II trials: They are not useful for eliminating unpromising therapeutic combinations or estimating the frequency and impact of serious toxicities. Conventional phase II trials also cannot “determine the characteristics of the cohort most likely to respond, especially in the absence of an identified biomarker,” he said.
Traditional phase II trials are inadequate in several ways, Dr. Kaplan explained. Their outcomes are unreliable when they use endpoints other than response rates (RRs), such as progression-free survival (PFS), which is now common in trials of targeted drugs. They often use historical controls that are out of date, and they suffer from selection bias, especially nonrandomized and single-center trials. Finally, conventional phase II trials often avoid intent-to-treat analyses in favor of evaluating “adequately treated patients,” although “patients who drop out early reflect clinical reality,” he said.
Dr. Kaplan called attention to the wide confidence intervals around the observed outcomes in studies that are based on limited numbers of patients recruited from a few centers. “These outcomes are not very easy to generalize; no wonder they do not accurately predict outcomes in conventional phase III trials. They also do not help us prioritize the most promising agents to go forward with in phase III,” he said.

Finally, equivocal or even negative outcomes in traditional phase II trials are often disregarded, and compounds move to phase III regardless, he observed. “Principal investigators and sponsors are sometimes anxious to continue development even when planned outcomes have not been reached,” Dr. Kaplan said.
Dr. Kaplan suggested that phase II trials could be worth keeping if they were modified to speed up the drug development process. In particular, he advocated larger cohorts enrolled from community centers and academic sites, randomization schemes, target validation and inclusion, and testing of biomarkers.
“While this will cost more, the data will be a better predictor of phase III outcomes,” he said.
But it is critical to design these randomized phase II trials so they are not just underpowered phase III trials, he emphasized. They should contain an intermediate endpoint to use as a prospective criterion for expanding to a phase III trial; clear study objectives for the phase II element; and specifications, such as a regulatory endpoint for definitive comparison, for any subsequent phase III trial.
This could be accomplished with a multi-arm, multi-stage design with the principal endpoint of survival, Dr. Kaplan said. An interim analysis of PFS would be performed, allowing for the weakest players to be discarded and the promising compounds to continue to the “mega-trial” with one or two arms, he said.
This multi-arm, multi-stage trial would require fewer patients and take less time to complete because it would be randomized from the start and would evaluate data concurrently and not sequentially (no need to wait for one result before proceeding). Also, there would be no delay between phase II and III. Finally, this type of trial would necessitate fewer grant applications.
“This is an efficient way to test multiple agents at the same time, to use fewer patients, and take less time, but it will take work to organize,” he conceded.
Traditional studies don’t have to be single arm
Before dismissing phase II trials as a waste of time and money, there needs to be a better understanding of the logistics of phase II trials, which is not as rigid as some would believe, Dr. Sobrero said.
“Traditional phase II trials continue to be useful for clinical practice and for drug development. Before we decide that phase II trials are a waste of time and money, we need to define a traditional phase II trial and define waste,” he said. While traditional phase II trials are often single-arm studies with RR as the primary endpoint, this is not always the case (see Figure). “[Phase II trials] don’t have to be single-arm studies to be traditional trials,” Dr. Sobrero said. “When you assess RR, you need sophisticated measurements. Regardless of what the [measurements] are, they all essentially measure tumor shrinkage. It doesn’t matter if you have categorical distinctions, or you look at the continuum, this is still a traditional phase II design.”
As for size, phase II trials are generally in the range of 30 to 60 patients, so studies of 200 or so patients are not in the definition, he said. But the definition should not exclude patient enrichment. “We know that HER2-positive patients with breast cancer have different outcomes from HER2-negative patients. This sort of patient enrichment occurs in many tumor types and is not excluded in phase II trials.”
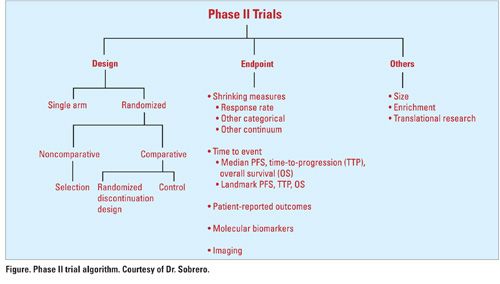
A trial in 40 molecularly selected patients with the endpoint being tumor shrinkage on a fountain graph would qualify as a traditional phase II trial, he said.
On the basis of this definition, Dr. Sobrero argued that phase II trials provide very valuable information for drug development and clinical practice. As an example, he pointed to a phase I/II study of PF-02341066, a novel c-met/ALK inhibitor, in 31 patients with advanced non-small-cell lung cancer who had the specific EML4-ALK translocation.
Twenty patients (65%) had partial responses to the single agent and another six had stable disease, yielding an overall disease control rate of 84% with this novel agent. Median PFS has not been reached after a median treatment duration of more than 24 weeks.
In another study, the oral multikinase inhibitor regorafenib (BAY 73-4506), evaluated as fourth- and fifth-line treatment for colorectal cancer, also produced responses or stable disease in about half the patients who received it.
“Tumor shrinkage in 50% of patients failing four lines of treatment is something special, in my opinion,” he said. “These were both traditional phase II studies: single arm, fewer than 60 patients, measuring response rate. And they produce valuable information. They show signals of activity. This is what you want from a phase II study.”
Response rates
Response rate, in fact, has been a highly relevant predictor of subsequent registration of new chemotherapy agents, as shown in 499 phase II (mostly traditional) trials of 46 drugs evaluated in more than 16,000 patients, he noted. For drugs that produced response rates of > 20%, 12 received regulatory approval. In addition, seven that produced response rates of 10% to 20% also received approval, compared with two where response rates were < 10%. Drugs that did not meet approval were those that produced no responses. “This had predictive value for the registration of agents,” Dr. Sobrero said.
“You may say that we don’t care about chemotherapy anymore in the era of biologics, but a recent review of trials of biologic agents concluded that response rates were still a good predictor of regulatory approval,” he said.
The review evaluated the relationship between trial outcomes and regulatory approval of targeted drugs. The authors evaluated 89 trials of 19 compounds. They found that the objective response was the primary or co-primary endpoint in 61 trials, 78% of which were single-arm studies and 70% of which had RR as the primary endpoint. The occurrence of an objective response to the targeted agent was significantly predictive of regulatory approval.
The authors concluded that phase II design for targeted agents was similar to that for cytotoxics and that RR was a useful endpoint for screening new biologics because it predicts for eventual success (J Clin Oncol 26:1346-1354, 2008).
But drug development is just one consideration when it comes to phase II trials; improvement in patient care is the other objective, Dr. Sobrero said. He reviewed issues of the Journal of Clinical Oncology over a four-month period to compile the number of phase II trials published in the journal and determine their relevance to his own practice.
Dr. Sobrero said that he found 26 phase II trials, including 11 studies of chemotherapy agents, 13 of biologics, and two of radiotherapy. Twenty were single-arm, traditional studies and six were randomized trials.
“My personal interpretation was that nine of the phase II trials were non-convincing, while 17 were useful to me,” he reported. “These included four nice transitional studies, seven studies that pointed to potential development of the drug, and six that currently helped me in my practice, all of which were traditional in design.”
Dr. Sobrero acknowledged that phase II trials are imperfect because they evaluate tumors that are classified according to an outdated system. He recommended that the cancer community should move away from the concept of traditional trials, but that phase II trials are still worthwhile.
“It’s a mistake to say traditional phase II trials are a waste of time and money,” he said. “They have led to the development of useful agents, both chemotherapeutic and biologic.”