COX-2 Inhibitors Added to Combination Chemotherapy in Advanced Colorectal Cancer
PORTLAND, Oregon-"Very preliminary data suggest that cyclooxygenase-2 (COX-2) inhibitors can be safely used in combination with irinotecan (Camptosar), and even more preliminary data suggest that such combinations are active in advanced colorectal cancer," reported Charles Blanke, MD. He explained that COX-2 is overexpressed in the majority of human colorectal cancers. Dr. Blanke is director of the GI Malignancy Program at the Oregon Cancer Institute in Portland.
PORTLAND, Oregon"Very preliminary data suggest that cyclooxygenase-2 (COX-2) inhibitors can be safely used in combination with irinotecan (Camptosar), and even more preliminary data suggest that such combinations are active in advanced colorectal cancer," reported Charles Blanke, MD. He explained that COX-2 is overexpressed in the majority of human colorectal cancers. Dr. Blanke is director of the GI Malignancy Program at the Oregon Cancer Institute in Portland.
Interest in COX-2 inhibitors for cancer treatment is based on the observation that COX-2 is expressed in many tumor tissues but not in most normal tissues. Use of nonselective, nonsteroidal anti-inflammatory drugs (NSAIDS) has been associated with a decrease in the mortality of colorectal cancer. In addition, COX-2 is known to play a role in premalignant polyp formation in familial adenomatous polyposis (FAP).
Dr. Blanke discussed work by Steinbach et al (New Eng J Med 342:1946, 2000) showing that celecoxib (Celebrex) at 400 mg BID decreased polyp burden in patients with FAP by 31% and polyp number by 28%. He pointed out that this relatively high dose was not associated with any excess adverse events in these patients. "It is important to note that arthritis-level doses of celecoxib, 100 mg BID, did not have any effect," he said.
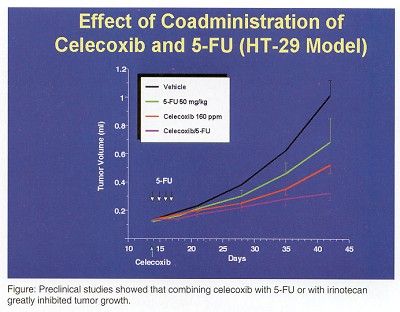
Inhibited Tumor Growth
COX-2 overexpression in the primary tumor is associated with an increase in metastases of colorectal cancer, particularly hematogenous metastases, and is independently associated with decreased disease-free survival in patients resected of large bowel malignancy (Br J Cancer 83[3]:324-8, 2000). "The authors of a Japenese trial concluded that COX-2 inhibitors might therefore be useful in preventing hematogenous metastasis," Dr. Blanke said.
Preclinical studies showed that combining celecoxib with 5-fluorouracil (5-FU) or with irinotecan greatly inhibited tumor growth.
Dr. Blanke is collaborating on a phase II trial of celecoxib with irinotecan and 5-FU/leucovorin in patients who have incurable, measurable colorectal cancer and have not previously been treated for metastatic disease. Patients must be 1 year past adjuvant therapy and must have received no prior agents except 5-FU with or without leucovorin.
The trial protocol calls for enrolling 54 patients, and Dr. Blanke said that 19 have been treated so far. "There has been little GI toxicity. It is possible that inhibiting thromboxane A with celecoxib might reduce irinotecan-related GI toxicity," he said. "There has also been a marked decrease in neutropenia, to about half what was expected." Efficacy cannot yet be estimated in this trial, but Dr. Blanke said that researchers have seen both some rapid progression and some early partial responses.
Dr. Blanke predicted that COX-2-inhibiting regimens are likely to have a role in the treatment of high-risk patients. He proposed that future trials incorporate steps to determine the molecular make-up of fresh tissue before and after treatment, including COX-2 expression, drug metabolic pathways, and downstream effects on apoptosis genes, genes involved in cell adhesion, repair mechanisms, and angiogenesis genes.