Curbing Potential Radiation-Induced Cancer Risks in Oncologic Imaging: Perspectives From the ‘Image Gently’ and ‘Image Wisely’ Campaigns
The purpose of this review is to provide the oncology community with knowledge about the doses used in medical imaging, radiation-induced cancer risks from imaging, and considerations to keep in mind when balancing imaging benefits and risks in pediatric and adult oncologic settings.
Figure 1: Extrapolation Models
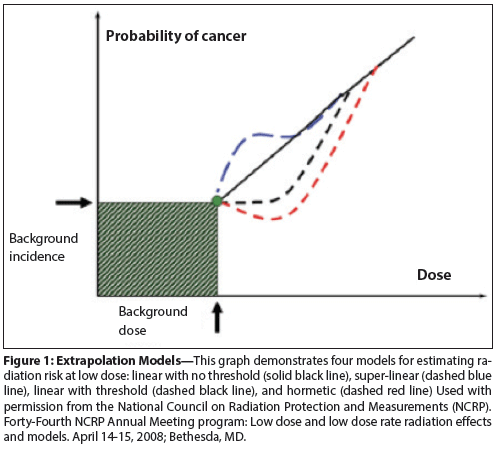
Figure 2: Logo for the ‘Image Gently’ Campaign

Figure 3: ‘Image Gently’ Advertisement

Figure 4: The Logo for the ‘Image Wisely’ Campaign

Medical imaging that uses ionizing radiation, such as CT, radiography, nuclear medicine, and fluoroscopy, is a cornerstone of the care of oncology patients and provides great benefit. Ionizing radiation at high doses is a known carcinogen. The exact degree of the risk of carcinogenesis from the lower doses of ionizing radiation used in medical imaging is less clear. The purpose of this review is to provide the oncology community with knowledge about the doses used in medical imaging, radiation-induced cancer risks from imaging, considerations to keep in mind when balancing imaging benefits and risks in pediatric and adult oncologic settings, dose reduction strategies, and the “Image Gently” and “Image Wisely” campaigns; the latter campaigns facilitate the translation of existing evidence into best practices for providers and patients.
Introduction
In recent years, there have been concerns about cancer risks from diagnostic medical imaging that depends on ionizing radiation.[1-14] The frequency of medical imaging-particularly computed tomography (CT)-has increased rapidly in the past decade, with approximately 70 million CT scans performed in the United States each year.[15] It has been speculated that in the US population, 29,000 radiation-induced cancers may develop as a result of the the CT scans done in a single year.[1] Risk projections such as these are drawn largely from a prospective, observational study of Japanese atomic bomb survivors. The resulting risk projections, summarized in the Biological Effects of Ionizing Radiation (BEIR) VII report, have been associated with some controversy when extrapolated to medical imaging, given the differences in exposure circumstances.[16-18] More recently, however, empiric evidence linking radiation exposure from imaging to increased cancer risks has emerged.[10,12] There is now a general consensus that while cancer risks from imaging are at most very low, they merit consideration in medical decision making.
When trying to achieve the optimal balance between the benefits of imaging tests such as CT, and the risks of radiation exposure in cancer patients, there are some unique considerations to bear in mind. First, cancer patients represent a highly diverse population, with varying ages, genders, and cancer-specific survival rates, three attributes that can substantially modify the magnitude of radiation-induced cancer risks.[16,19] Second, cancer patients commonly receive multiple medical imaging tests, particularly CT and positron emission tomography (PET)/CT scans, in the contexts of diagnosis and staging, treatment, and post-treatment surveillance.[14,20] Third, cancer treatments such as chemotherapy and comorbidities such as lymphoproliferative disease and secondary cancers may work synergistically with the ionizing radiation from imaging studies to impact outcomes. Finally, because imaging benefits are typically tied to intermediate-and not long-term-patient outcomes, very few studies that yield Level I evidence (ie, randomized controlled trials) have been done that test the effects of imaging protocol differences on progression-free or cancer-specific survival rates.[21-23] Without a strong evidence base to inform the risks of not imaging, or of “skipping” a protocol-driven CT scan, it can be difficult to accurately judge the trade-offs involved in bypassing imaging due to concerns about radiation exposure.
With so many factors to consider-and a dearth of evidence to provide guidance-decisions surrounding the
use of imaging in cancer care can be difficult. In this review, our goal is to provide the oncology community with knowledge about: 1) the nature of radiation-induced cancer risks from medical imaging that uses ionizing radiation; 2) dose assessment and doses of diagnostic imaging studies; 3) considerations to keep in mind when balancing imaging benefits and risks in pediatric and adult oncologic settings; 4) techniques for radiation dose reduction; and 5) the “Image Gently” and “Image Wisely” campaigns, which facilitate translation of existing evidence into best practices for providers and patients.[3,6]
Radiation-Induced Cancer Risks From Imaging: Current Evidence
Supported largely by data from the Japanese atomic bomb survivor cohort, the BEIR VII committee has endorsed a linear no-threshold (LNT) model to explain the relationship between radiation exposure and cancer risk for all radiation-induced solid cancers, and a linear-quadratic (ie, upward-sloping) model to explain the exposure-risk relationship for leukemias (Figure 1).[16] Given the lesser contribution of leukemias relative to solid cancers when all radiation-induced cancers are considered collectively, the LNT model dominates translation of BEIR VII findings to practice. Moreover, among all radiation-risk models proposed and used for projecting cancer risks from imaging, the LNT model remains the most widely accepted to date.[1,13,14,17]
Primary alternative models also merit mention, including the linear-threshold and hormesis models in particular.[18,24-26] The linear-threshold model asserts that below a specific threshold-one that is not known with certainty but that is generally considered to be above that of any single imaging study-there is no risk incurred.[24,25] This model is grounded in the biologic assumption that DNA repair mechanisms nullify the cellular injury incurred from low levels of radiation exposure.[24,25] Above this threshold, the exposure-risk relationship is considered linear, as in the LNT model. In a hormesis model, low levels of radiation exposure are presumed to have an anticarcinogenic (favorable) effect on cancer risk.[25,26] The threshold beyond which exposures result in cancer risks rather than health benefits is not known, but as with the linear-threshold model, it is considered to be above that of any single imaging study. Notably, despite proponents of both alternative models, neither has gained traction commensurate to that of the LNT model.
Repeated imaging tests-and associated radiation exposures-are oftentimes the rule in cancer care. How should risks from cumulative, past exposures be approached when making prospective imaging decisions? Invoking the LNT model, risks are expected to accumulate over a patient’s lifetime, in a manner that adheres to LNT principles. Notably, adaptation of the LNT model to account for risk accumulation implies that prior exposures do not influence future risks, a mathematical inference of the linear relationship between exposure and risk.[27-30] To consider how this concept might apply in practice, suppose a young cancer survivor, who has a remote history of multiple chest, abdomen, and pelvis surveillance CT scans, presents with nausea, vomiting, and abdominal pain. At the same time, a patient with no exposure (or cancer) history, but who is otherwise identical, presents with the same symptoms. Applying LNT principles, if the anticipated benefit of a CT scan is the same for the two patients, and if the risk-benefit ratio favors a CT scan in the patient with no prior exposure history, then the CT scan is also indicated for the patient with an exposure history: the risk of the CT scan is the same either way.[27-30]
Conversely, if it were the case that this application of the LNT model were incorrect, and that each additional exposure resulted in a slightly heightened “per exposure” risk, then it would be logical to consider the burden of risks from past exposures when making a current imaging decision. Shuryak and colleagues have proposed a distinct analytic approach for evaluating the age dependency of radiation-induced cancer risks.[31] Their model incorporates not only radiation-induced cancer induction, but also radiation-induced promotion of premalignant cells.[31] Applying these principles (of induction and promotion) to settings of multiple exposures suggests that prior exposures could influence risks from current exposures. However, the degree to which a prior exposure would, in this setting, influence the magnitude of a future risk is not known. Given the uncertainty that underlies existing evidence in this field-and given the relatively small magnitude of cancer risks from imaging in general-past radiation exposures should not be factored into current imaging decisions. This is not to say that past imaging history is not important. Obviously, findings on prior studies are important and may inform the potential benefit of repeat imaging studies.
Regarding anticipated future cumulative exposures (eg, for planned post-treatment cancer surveillance), oncologic subspecialties are urged to complete corresponding imaging risk-benefit analyses for target patient populations “up front,” rather than during the course of a patient’s care. This should ideally be done using a combination of available evidence and expert consensus, as is done, for example, during formulation of National Comprehensive Cancer Network guidelines. While providers may elect to adjust, in some cases, the dose, frequency, or modalities of imaging schedules at the individual patient level, this should be done with caution. In many settings, the risks that are clinically weighed against one another are small (cancer recurrence vs radiation-induced cancer risks). Therefore, even a busy provider is unlikely to build an experience base that will allow for rational imaging protocol adjustments at the provider level. The importance of adhering to consensus guidelines, as a result, must be emphasized.
Imaging Modalities and Dose Assessment
Medical imaging is critical in the care of cancer patients. Imaging detects disease and response to treatment, supports surgical decisions, aids in disease staging, and allows monitoring of recurrence and intercurrent illness.[22] These benefits of medical imaging should be weighed against the relative risk of imaging. The risks associated with diagnostic imaging consist mostly of cancer (a stochastic risk); rarely, interventional radiation can result in tissue reactions (also called deterministic effects), such as skin erythema and ulceration, but the latter are and should be very rare.[32] Radiation risk for individual modalities that use ionizing radiation, including radiography, fluoroscopy (including that used in interventional radiology), CT, and nuclear imaging, as well as hybrid technology (especially PET/CT), depends primarily on the dose delivered and its biologic impact on radiated tissues. The risk will also depend on factors previously described, including age, gender, body morphology (eg, obesity), genetic or syndromic susceptibility, and protracted vs acute exposure; however, these factors are beyond the purposes of this discussion. Since radiation dose is the primary quantity, it is useful to review how these doses are measured and estimated as a background for understanding the significance of and challenges associated with relative differences in radiation doses delivered to children and adults.
Currently, there is no simple method that can accurately measure the radiation dose absorbed by an individual patient from radiography and CT scans. For this reason, doses to patients for many imaging tests are estimates. Dose measurements or estimations are performed in different ways for the modalities that consist primarily of radiation released from an external source (radiography, fluoroscopy, CT) or from internally administered agents (nuclear imaging). Comparing these different metrics for dose is difficult, especially when assessing risks. Hence, what is currently used as a common dose “currency” is the effective dose, which affords comparison between the different modalities, and between different regional exposures, although there are caveats regarding the use of effective dose in medical imaging.[27,29] Different modalities and regional exposures and organ doses are converted to an “equivalent” whole-body dose. The effective dose unit is the Sievert (SV), with doses for diagnostic imaging usually in the range of milli-Sieverts (mSv). It is important to remember that regional examinations can carry quite different doses depending on what organs are exposed. For example, a pelvis CT may have an effective dose of 10.0 mSv, and a chest CT an effective dose of 1.0 mSv. While the effective dose in the former is 10× that of the latter, the breast exposure, hence organ dose, in a young women is essentially zero for the pelvis CT, but depending on the technique used, may be around 25 mGy for the chest CT, with attendant potential risk that is greater despite the much lower effective dose.
There is additional debate concerning the issue of acute vs protracted exposures, and this is perhaps especially pertinent in oncologic imaging. For example, consider this question: does a single CT with an effective dose of 50 mSv carry the same overall potential risk as 5 CT examinations of 10 mSv each, spread out over 6 months? Whether there is a cumulative effect from multiple exposures-either from the same modality or from different modalities-is uncertain. However, the simplest model posits that cumulative dose carries the same risk as an acute exposure (adjusting for factors such as age at exposure in the protracted case).
Radiation Risk Considerations in Oncologic Imaging Decisions
The oncologic and imaging communities strive to implement imaging in ways that both optimize patient outcomes associated with primary cancers and minimize risks of secondary radiation-induced cancers. Cancer, however, is a heterogeneous disease, and the characteristics of affected patients can be highly variable even within one stage, grade, and tumor subtype. These variables modify the magnitude and relative importance of radiation-induced cancer risks, but there is no universally applicable approach to related imaging decisions. Therefore, an understanding of factors that may affect patients’ radiation-induced cancer risks-and of how to best weigh these risks-is very important for achieving high-quality imaging decisions at the individual patient level.
First, cancer risks from radiation exposure are expected to vary with age and gender. For example, 20-year-old men exposed to a 10-mSv imaging study (an effective dose associated with a typical abdominopelvic CT in an adult) are projected to have a 98/100,000 lifetime attributable risk (LAR) of developing a radiation-induced cancer.[16] The corresponding LAR for 20-year-old women is projected to be 165/100,000. In comparison, LAR estimates for the same exposure level in 80-year-olds are much lower (17/100,000 in men, and 21/100,000 in women).[16] As a result, modification of imaging frequency or radiation dose may not be warranted for older patients, while great caution should be exercised in younger patients and children as radiosensitivities are clarified.[33] Children diagnosed with cancer represent a unique patient population at particular risk for adverse effects of exposure to ionizing radiation, for several reasons. Overall survival in this pediatric cohort now exceeds 80%, with some cancers now associated with a greater than 90% survival rate. Thus, the majority of children treated for cancer survive into adulthood.[22] The risk period for developing second cancers related to cumulative exposure to ionizing radiation is therefore present over a longer lifetime compared with adults.
Second, mortality risks from a potential radiation-induced cancer must be considered in the context of the mortality risks from a patient’s primary cancer. Following an exposure, radiation-induced cancer risks begin to manifest after relatively long latency periods; latency periods are estimated to be 2 to 5 years, at a minimum, for radiation-induced leukemias, and 5 to 10 years for radiation-induced solid cancers.[16,34] Thereafter, risks of radiation-induced cancers likely continue, at low levels, for the remainder of patients’ lives.[16] This means that if a patient’s life expectancy is less than 2 years, radiation-induced cancer risks should not factor into imaging decisions. Even for patients who may live longer, the competing risk from their primary cancer still-on average-diminishes the relative risk of their developing a radiation-induced cancer.[19,20] Brenner and colleagues recently used biostatistical methods to compute the effects of competing health risks on radiation-induced cancer risks from imaging.[19] They demonstrated that the risk of developing a radiation-induced lung cancer from CT decreased by 8% in 70-year-old patients with stage 0/I colon cancer, and by 92% in 70-year-old patients with stage IV colon cancer.[19]
Third, when making imaging decisions in cancer patients, providers must consider the timing of proximate health risks-in particular, those related to patients’ primary cancers (eg, recurrence) and treatment complications-relative to more distant radiation-induced cancer risks.[35] For example, consider a 20-year-old cancer patient who, in the middle of chemotherapy and associated neutropenia, presents to his oncologist with abdominal pain. When deciding whether to order a CT scan or an ultrasound to evaluate for colitis, it is important to consider both the patient’s young age and his higher risk of developing a radiation-induced cancer in the future, and the more immediate, competing threat to the patient’s health that imaging may help avert, which is occurring now. An ultrasound examination could be chosen instead of CT if uncomplicated colitis was clinically expected.[36] Conversely, if the patient appeared acutely ill or rapidly decompensating, and there were additional diagnostic concerns beyond colitis, then a CT scan would likely be preferable, since the benefits of CT (ie, preventing a potentially fatal missed diagnosis) would outweigh more distant cancer risks associated with low levels of radiation exposure.
Fourth, imaging goals and benefits differ greatly across cancer settings, and these differences must also be considered carefully when making decisions about how to best use imaging. For example, for colon cancer patients who undergo CT surveillance after potentially curative surgery, implementing radiation dose-reduction techniques that may compromise image quality is not advisable, even in younger patients. The conspicuity of liver lesions-which requires the resolution achievable with CT-is of critical importance in this population, since secondary hepatic resections can still achieve cure.[37] Conversely, in patients with stage I testicular cancer, who are oftentimes surveyed with CT multiple times per year after orchiectomy, an isolated liver recurrence is extremely unlikely; most recurrences are within lymph node chains that remain readily visible even with substantial reductions in radiation exposure. In this cancer setting, use of dose-reduction techniques is more likely to succeed in optimizing patient outcomes.[38]
In contrast to adult patients, the vast majority of children undergoing therapy for cancer are enrolled in trials with treatment protocols that dictate the type, frequency, and sometimes techniques of imaging studies to be performed. Such studies attempt to standardize, at least to a degree, disease staging, response assessment, and follow-up for disease recurrence. Thus, in the design of protocols, cumulative patient exposure to ionizing radiation should be considered while optimizing acquisition of needed information.[22]
Opportunities in Diagnostic Imaging for Managing Radiation Exposure
A basic tenet of imaging is that only necessary examinations, including CT examinations, should be performed. This is the justification element of radiation protection. However, when a CT examination is warranted, then the examination should be performed appropriately. This is the optimization element of radiation protection. Avoiding unnecessary examinations is one dose-reduction technique; in the following we focus on technique and technical aspects of dose management, including dose reduction in CT. More detailed discussion of dose-reduction strategies for both adult and pediatric CT can be found in several sources.[39-44] The same dose-reduction strategies often apply to both adults and children. These strategies are the shared responsibility of radiologists and other imaging providers, technologists, and medical and health physicists. Moreover, in a broader sense, because education and regulation and regulatory guidance are aspects of dose reduction, industry, regulatory agencies, and professional societies are also stakeholders.
In both pediatric and adult CT, patient preparation is critical to assure proper performance. This preparation includes removal of devices that may cause compromising artifacts, and awareness of internal devices, such as clips, stents, and orthopedic hardware, that negatively impact image and study quality. Patient positioning is also important. For brain CT, care should be taken to minimize lens exposure. Optimal use of the dose reduction technology summarized below may only be achieved with correct patient centering. In children, additional considerations include appropriate sedation, coaching when necessary for breath holding or keeping still, and attention to technique for intravenous iodinated contrast administration. Unless contrast administration is optimized, image and study quality may suffer and dose-reduction benefits may be compromised.
The “Image Gently” and “Image Wisely” campaigns suggest employing the following measures when performing imaging examinations. First, only the necessary region should be covered. Multiple phases, such as the two phases in a pre- and post-contrast chest CT (sometimes referred to as dual scanning), can provide additional and necessary information based on differences in contrast enhancement. Multi-phase exams tend to be more utilized in adults; however, even in adults, they should only be used when there is a clear medical benefit and when an alternative imaging modality, such as MRI, is not available. In pediatric CT, multi-phase scans should be used sparingly-only in a small percentage of cases where body CT, for example, is required.[45] Each phase, if there is no change in CT settings, will increase the dose, multiplying it by the number of examinations performed. In general, doses can be relatively lower for chest and airway CT, skeletal CT, and some CT angiography, as well as for follow-up examinations, for which the level of detail in the original CT scan (eg, higher spatial resolution) may not be necessary. Occasionally, very limited CT evaluation of a region may be warranted if there is a specific clinical indication. Examples include follow-up of a possible pulmonary nodule, or perhaps empyema follow-up in an immunocompromised patient.
The ‘Image Gently’ campaign
In 2001, Paterson et al found that children were often receiving the same dose of radiation as adults when undergoing CT scans.[46] This realization, along with the fact that for most cancers children are considered more vulnerable to the potential effects of ionizing radiation than adults, led to the formation of the Alliance for Radiation Safety in Pediatric Imaging (“the Alliance”) in 2007.[47] Founded by the Society for Pediatric Radiology (SPR), the American Society of Radiologic Technologists (ASRT), the American College of Radiology (ACR), and the American Association of Physicists in Medicine (AAPM), the primary goal of the Alliance is to raise awareness, provide education, and advocate for radiation protection for children undergoing medical imaging. Today, the Alliance is a coalition of 76 medical professional societies and agencies, 22 of which are international.
The mission of the Alliance is to “raise awareness of the need to adjust radiation dose when imaging children. The ultimate goal of the Alliance is to change practice.” [47] The Alliance created the “Image Gently” campaign (Figure 2), and uses “social marketing” to accomplish its mission.[48] Social marketing is defined as “the application of commercial marketing technologies to the analysis, planning and execution and evaluation of programs designed to influence the behavior of a target audience in order to improve their personal welfare and that of the society for which they are a part.”[49] The target audience for the “Image Gently” campaign has expanded from the original triad of radiologists, radiologic technologists, and medical physicists, and now includes parents and the public, government and agencies, referring physicians, and manufacturers of imaging equipment. The Alliance uses its website (www.imagegently.org), advertisements, posters, promotional items, social media, and the internal communication of its member societies to reach its target audience.â©In addition, the Alliance participated in the development of the Size-Specific Dose Estimate, a new patient dose estimate. This measurement is accurate within 10% to 20% of patient dose[50] and is expected to be adopted by CT manufacturers. The Alliance has conducted five campaigns (Figure 3). Other resources from the Alliance include the Image Gently “universal protocols” that include specific guidelines for size-based pediatric CT protocols that can be used with any CT scanner, regardless of manufacturer, number of detectors, or scanner age. These protocols are derived from the individual facilities’ protocols used for adult-sized patients.[51]
The ‘Image Wisely’ campaign
Whereas the “Image Gently” campaign is focused on children, “Image Wisely” is a social marketing campaign to raise awareness about adult radiation protection in medical imaging (Figure 4).[3] It evolved from the Joint Task Force on Adult Radiation Protection of the the Radiological Society of North America (RSNA) and the ACR, with the intent of building on the success of the “Image Gently” campaign at raising awareness about the need for radiation protection in children. The adult initiative recognized the importance of including the ASRT and the AAPM as key partners, as did “Image Gently.”
The original plan was that the “Image Wisely” campaign would be executed in three distinct phases, ordered according to the magnitude of radiation exposure (in the US population) from the various medical imaging modalities.[52] The greatest population exposure from medical imaging comes from CT, followed sequentially by nuclear medicine and radiography/fluoroscopy. “Image Wisely” was launched at the annual meeting of the RSNA in November 2010 for CT, and the campaign’s new website (www.imagewisely.org) was unveiled at the same time. The website collects and catalogs premier educational content for imaging professionals; presents frequently asked questions geared towards referring practitioners; and provides links to www.RadiologyInfo.org, where valuable patient-focused material and downloadable patient medical imaging record cards can be obtained. In addition, because radiation management methodology is often device-specific, the website assembles links to micro-sites developed and maintained by manufacturers to provide information on radiation safety for their devices. Nuclear medicine information was added to the website in 2011; fluoroscopy is planned for 2014.
Conclusions and Future Directions
Diagnostic imaging has a central and invaluable role in the care of oncology patients. Much of this imaging consists of modalities that use low-level ionizing radiation with debatable risks for cancer induction. Oncology patients have some legitimate concerns, given the amount of diagnostic imaging that may be performed, among other considerations. Therefore, it is necessary for the community caring for this population to understand doses delivered; how doses are estimated; what is understood about risks and limitations in determining these risks; factors to consider when weighing benefits vs risks; and strategies for dose management, including those promulgated through such campaigns as the “Image Gently” and “Image Wisely” campaigns.
Financial Disclosure:Dr. Pandharipande receives research funding from the Medical Imaging and Technology Alliance. The other authors have no financial interest or other relationship with the manufacturer of any product or provider of any service mentioned in the article.
References:
1. Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-7.
2. Brenner DJ, Hall EJ. Computed tomography-an increasing source of radiation exposure. N Engl J Med. 2007;357:2277-84.
3. Brink JA, Amis ES Jr. Image Wisely: a campaign to increase awareness about adult radiation protection. Radiology. 2010;257:601-2.
4. Brink JA, Goske MJ, Patti JA. Informed decision making trumps informed consent for medical imaging with ionizing radiation. Radiology. 2012;262:11-4.
5. Frush DP, Applegate K. Computed tomography and radiation: understanding the issues. J Am Coll Radiol. 2004;1:113-9.
6. Goske MJ, Applegate KE, Boylan J, et al. The ‘Image Gently’ campaign: increasing CT radiation dose awareness through a national education and awareness program. Pediatr Radiol. 2008;38:265-9.
7. Goske MJ, Frush DP, Schauer DA. Image Gently campaign promotes radiation protection for children. Radiat Prot Dosimetry. 2009;135:276.
8. Harvey HB, Pandharipande PV. The federal government's oversight of CT safety: regulatory possibilities. Radiology. 2012;262:391-8.
9. Kaste SC. Imaging challenges: a US perspective on controlling exposure to ionizing radiation in children with cancer. Pediatr Radiol. 2009;39 Suppl 1:S74-9.
10. Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360.
11. Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167:700-7.
12. Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499-505.
13. Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078-86.
14. Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology. 2009;251:175-84.
15. Mettler FA Jr, Bhargavan M, Faulkner K, et al. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources-1950-2007. Radiology. 2009;253:520-31.
16. National Research Council (US) Advisory Committee on the Biological Effects of Ionizing Radiations. Health risks from exposure to low levels of ionizing radiation, BEIR VII phase 2. Washington DC: National Academy of Sciences; 2006.
17. Little MP, Wakeford R, Tawn EJ, et al. Risks associated with low doses and low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology. 2009;251:6-12.
18. Tubiana M, Feinendegen LE, Yang C, Kaminski JM. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology. 2009;251:13-22.
19. Brenner DJ, Shuryak I, Einstein AJ. Impact of reduced patient life expectancy on potential cancer risks from radiologic imaging. Radiology. 2011;261:193-8.
20. Zondervan RL, Hahn PF, Sadow CA, et al. Body CT scanning in young adults: examination indications, patient outcomes, and risk of radiation-induced cancer. Radiology. 2013;267:460-9.
21. Rustin GJ, Mead GM, Stenning SP, et al. Randomized trial of two or five computed tomography scans in the surveillance of patients with stage I nonseminomatous germ cell tumors of the testis: Medical Research Council Trial TE08, ISRCTN56475197-the National Cancer Research Institute Testis Cancer Clinical Studies Group. J Clin Oncol. 2007;25:1310-5.
22. Weiser DA, Kaste SC, Siegel MJ, Adamson PC. Imaging in childhood cancer: a Society for Pediatric Radiology and Children's Oncology Group Joint Task Force report. Pediatr Blood Cancer. 2013;60:1253-60.
23. Kaste SC, Brady SL, Yee B, et al. Is routine pelvic surveillance imaging necessary in patients with Wilms tumor? Cancer. 2013;119:182-8.
24. Tubiana M. Dose-effect relationship and estimation of the carcinogenic effects of low doses of ionizing radiation: the joint report of the Academie des Sciences (Paris) and of the Academie Nationale de Medecine. Int J Radiat Oncol Biol Phys. 2005;63:317-9.
25. Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci USA. 2003;100:13761-6.
26. Jolly D, Meyer J. A brief review of radiation hormesis. Australas Phys Eng Sci Med. 2009;32:180-7.
27. Durand DJ. A rational approach to the clinical use of cumulative effective dose estimates. Am J Roentgenol. 2011;197:160-2.
28. Eisenberg JD, Harvey HB, Moore DA, et al. Falling prey to the sunk cost bias: a potential harm of patient radiation dose histories. Radiology. 2012;263:626-8.
29. Pandharipande PV, Eisenberg JD, Avery LL, et al. Journal club: how radiation exposure histories influence physician imaging decisions: a multicenter radiologist survey study. Am J Roentgenol. 2013;200:1275-83.
30. Durand DJ, Dixon RL, Morin RL. Utilization strategies for cumulative dose estimates: a review and rational assessment. J Am Coll Radiol. 2012;9:480-5.
31. Shuryak I, Sachs RK, Brenner DJ. Cancer risks after radiation exposure in middle age. J Natl Cancer Inst. 2010;102:1628-36.
32. Hricak H, Brenner DJ, Adelstein SJ, et al. Managing radiation use in medical imaging: a multifaceted challenge. Radiology. 2011;258:889-905.
33. United Nations Scientific Committee on the Effects of Atomic Radiation. Sources, effects and risks of ionizing radiation, vol II, scientific annex B. New York: United Nations; 2013.
34. Office of Radiation and Indoor Air, US Environmental Protection Agency. Modifying EPA radiation risk models based on BEIR VII; draft white paper. 2006 Aug 1. Available from: http://www.epa.gov/radiation/docs/assessment/white-paper8106.pdf. Accessed February 6, 2014.
35. Pandharipande PV, Eisenberg JD, Lee RJ, et al. Patients with testicular cancer undergoing CT surveillance demonstrate a pitfall of radiation-induced cancer risk estimates: the timing paradox. Radiology. 2013;266:896-904.
36. McCarville MB. Evaluation of typhlitis in children: CT versus US. Pediatr Radiol. 2006;36:890-1.
37. Tsikitis VL, Malireddy K, Green EA, et al. Postoperative surveillance recommendations for early stage colon cancer based on results from the clinical outcomes of surgical therapy trial. J Clin Oncol. 2009;27:3671-6.
38. O’Malley ME, Chung P, Haider M, et al. Comparison of low dose with standard dose abdominal/pelvic multidetector CT in patients with stage 1 testicular cancer under surveillance. Eur Radiol. 2010;20:1624-30.
39. Strauss KJ, Goske MJ, Kaste SC, et al. Image Gently: ten steps you can take to optimize image quality and lower CT dose for pediatric patients. Am J Roentgenol. 2010;194:868-73.
40. Tamm EP, Rong XJ, Cody DD, et al. Quality initiatives: CT radiation dose reduction: how to implement change without sacrificing diagnostic quality. Radiographics. 2011;31:1823-32.
41. Kalra MK, Maher MM, Toth TL, et al. Strategies for CT radiation dose optimization. Radiology. 2004;230:619-28.
42. Khong PL, Frush D, Ringertz H. Radiological protection in paediatric computed tomography. Ann ICRP. 2012;41:170-8.
43. Frush D. CT radiation and children: Strategies for mitigating radiation exposure in children. J Surg Rad. 2011;2:358-63.
44. Nievelstein RA, van Dam IM, van der Molen AJ. Multidetector CT in children: current concepts and dose reduction strategies. Pediatr Radiol. 2010;40:1324-44.
45. Goske MJ, Strauss KJ, Coombs LP, et al. Diagnostic reference ranges for pediatric abdominal CT. Radiology. 2013;268:208-18.
46. Paterson A, Frush DP, Donnelly LF. Helical CT of the body: Are settings adjusted for pediatric patients? Am J Roentgenol. 2001;176:297-301.
47. Goske MJ, Applegate KE, Bell C, et al. Image Gently: providing practical educational tools and advocacy to accelerate radiation protection for children worldwide. Semin Ultrasound CT MR. 2010;31:57-63.
48. Goske MJ, Applegate KE, Boylan J, et al. The Image Gently campaign: working together to change practice. Am J Roentgenol. 2008;190:273-4.
49. Andreasen A. The role of social marketing. In: Andreasen A. Social marketing in the 21st century. Thousand Oaks, CA: Sage; 2006. p. 87-107.
50. Boone JM, Strauss KJ, Cody DD, et al. Size-specific dose estimates (SSDE) in pediatric and adult body CT examinations. AAPM Report No. 204; 2011. ISBN: 978-1-936366-08-8, ISSN: 0271-7344. Available from: http://www.aapm.org/pubs/reports/RPT_204.pdf. Accessed October 2, 2011.
51. Strauss KJ. Image Gently universal protocols; 2008. Available from: http://www.pedrad.org/associations/5364/files/Protocols.pdf. Accessed 2 October 2, 2011.
52. National Council on Radiation Protection and Measurement (NCRP) Report 160: Ionizing radiation exposure of the population of the United States; 2009. Available from: http://www.ncrponline.org/Publications/Press_Releases/160press.html. Accessed February 6, 2014.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.