Metastatic Relapse After Initial Clinical Stage I Testicular Leydig Cell Tumor
A 70-year-old, otherwise healthy man presented to his primary care provider for an annual checkup, at which time a nontender right testicular mass was noted. He denied any symptoms, and his serum alpha-fetoprotein and beta-human chorionic gonadotropin levels were normal.
Figure 1: Histopathology Demonstrating LCT
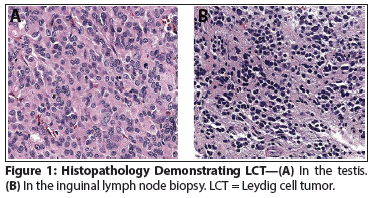
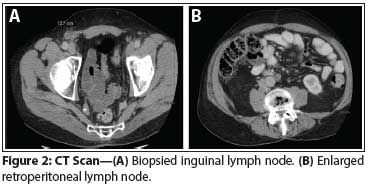
Figure 2: CT Scan
The Case:A 70-year-old, otherwise healthy man presented to his primary care provider for an annual checkup, at which time a nontender right testicular mass was noted. He denied any symptoms, and his serum alpha-fetoprotein and beta-human chorionic gonadotropin levels were normal. A right radical inguinal orchiectomy was subsequently performed. The pathology report demonstrated a 2.1-cm lesion consistent with a Leydig cell tumor (LCT), which was noted to have moderate mitotic activity (4–5 mitoses per high-power field [HPF]), as well as invasion of the spermatic cord (pT3) (Figure 1A). Of note, no lymphovascular invasion (LVI), cellular atypia, or tumor necrosis was identified. Staging CT scans of his chest, retroperitoneum, and pelvis were negative. Given the LCT histology, further testing was done, and per the report, there was no elevation of serum testosterone or inhibin levels at that time. Pertinently, the patient had no symptoms of virilization or feminization. In summary, the patient’s tumor was determined to be a clinical stage IB testicular LCT.
While on periodic surveillance with physical exams and imaging, a 1.2-cm right inguinal lymph node and a 1-cm left para-aortic lymph node were discovered on a CT scan 2 years after initial presentation. For unknown reasons, no action was taken immediately, but a follow-up CT scan 3 months later demonstrated an increase in the size of the inguinal and para-aortic nodes (Figure 2). This prompted percutaneous biopsy of the inguinal node, which was consistent with metastatic LCT (Figure 1B). At this time the patient’s serum testosterone was rechecked and was 835 ng/dL, despite his monorchid status and his age of 72 years.
He was then referred to our tertiary medical center for consideration of chemotherapy and/or surgery. Multidisciplinary discussion with the patient ensued, with consideration of chemotherapy using regimens described in the literature, which are mostly extrapolated from the treatment of testicular germ cell tumors. While these have failed to demonstrate long-term cure, they have demonstrated the ability to induce partial volumetric responses. Additionally, surgery in the form of a right inguinal and bilateral retroperitoneal lymph node dissection (RPLND) was considered, given the chemorefractory nature of LCTs. This discussion resulted in the patient electing to undergo right inguinal and bilateral RPLND.
Discussion
Testicular stromal tumors (TSTs) make up less than 5% of all primary testicular tumors[1]; however, LCTs are the most common subtype, comprising approximately 75% of all TSTs.[2] Prior historical series indicate that the vast majority-approximately 90%-of testicular LCTs behave in a benign manner and are cured with orchiectomy alone.[3] Unfortunately, 10% are metastatic at diagnosis or relapse after initial presentation as clinical stage I disease. While metastatic disease is a definitive indicator of malignancy, some pathologic criteria from the orchiectomy specimen have been reported to correlate with malignant behavior.[3,4] These “high-risk” criteria are most commonly considered to be:
•Larger tumor dimension (> 5 cm).
•Positive surgical margins or spread outside the testis.
•LVI.
•Cellular atypia.
•Increased mitotic rate (≥ 5 mitoses per HPF).
In addition, these tumors, because of the nature of their physiology, may be hormonally active, presenting with elevated serum testosterone, estrogen, or inhibin levels; the elevated hormone levels may correlate with symptoms such as gynecomastia or hypervirilization.
Regardless of these pathologic correlations, there still remains a lack of evidence-based data to predict which patients initially diagnosed with clinical stage I disease will relapse. This is important in clinical management because there is no effective systemic chemotherapy or radiotherapy known to be curative in metastatic disease. This prompts the germane question of whether aggressive adjuvant surgical treatment with primary RPLND in high-risk patients is beneficial and prevents future relapse, since the development of metastatic disease is not salvageable with subsequent therapy. Gohji et al presented three clinical stage I testicular LCT cases in which the patients underwent primary RPLND and were found to have pathologic stage I disease.[5] They reported no recurrences after a mean follow-up of 29 months after RPLND. Similarly, Peschel et al presented a series of six adult men with clinical stage I LCT who were managed with laparoscopic RPLND and were all found to be pathologic stage I; none experienced relapse at a mean of 12 months after RPLND.[6] In contrast, Mosharafa et al made slightly different observations in their Indiana University study in which 13 patients with clinical stage I TST underwent primary RPLND; -of these patients, four had pathologic stage II disease at the time of RPLND.[7] While no recurrences were observed in the nine pathologic stage I patients, all four patients found to have pathologic stage II tumors suffered subsequent disease progression. This calls into question whether RPLND is less a therapeutic intervention in this setting, and perhaps more a staging tool.
Our patient’s case highlights two issues in the management of testicular LCTs. One, should such a patient have been considered for a primary RPLND, given his risk factors of pT3 status and increased mitotic activity? The question is complicated in this case by the fact that the sites of recurrence in our patient (inguinal lymph node and contralateral retroperitoneal lymph node) would not have been removed using a standard unilateral template RPLND. The second issue is how to manage the metastatic disease once it is identified. Mosharafa et al described their multimodal management of metastatic TSTs and reported on eight patients with stage II or III disease. They observed that six of eight patients died of disease progression, with a mean survival of 2.4 years.[7] While systemic chemotherapy (bleomycin, etoposide, cisplatin) was administered to these patients with recurrence, they reported that the response to therapy was partial and of short duration. The two surviving patients were disease-free after 8 and 13 months, respectively. Their conclusions were that the response seen from chemotherapy is not curative, and that while surgery may render the patient disease-free temporarily, there is a high likelihood of recurrence from unrecognized micrometastatic disease. Despite a lack of robust data supporting surgical resection in the setting of metastatic disease, the fact that chemotherapy or radiation is not curative leaves surgical resection as the only treatment capable of rendering patients disease-free, even if only temporarily.
Conclusion
As highlighted, the absence of evidence for any specific therapy makes the management of metastatic LCT difficult. Given the relative rarity of this disease, a prospective, multi-institutional cooperative group study would likely be necessary to determine the role of single-agent or multimodality therapy in this setting. Similarly, further investigation is warranted into the role of adjuvant RPLND in patients with high-risk clinical stage I disease, since salvage therapies offer less than ideal outcomes.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Dilworth JP, Farrow GM, Oesterling JE. Non-germ cell tumors of testis. Urology. 1991;37:399-417.
2. Risk MC, Porter CR. Management of non-germinal testicular tumors. World J Urol. 2009;27:507-12.
3. Acar C, Gurocak S, Sozen S. Current treatment of testicular sex cord-stromal tumors: critical review. Urology. 2009;73:1165-71.
4. Cheville JC, Sebo TJ, Lager DJ, et al. Leydig cell tumor of the testis: a clinicopathologic, DNA content, and MIB-1 comparison of nonmetastasizing and metastasizing tumors. Am J Surg Pathol. 1998;22:1361-7.
5. Gohji K, Higuchi A, Fujii A, Kizaki T. Malignant gonadal stromal tumor. Urology. 1994;43:244-7.
6. Peschel R, Gettman MT, Steiner H, et al. Management of adult Leydig-cell testicular tumors: assessing the role of laparoscopic retroperitoneal lymph node dissection. J Endourol. 2003;17:777-80.
7. Mosharafa AA, Foster RS, Bihrle R, et al. Does retroperitoneal lymph node dissection have a curative role for patients with sex cord-stromal testicular tumors? Cancer. 2003;98:753-7.