Neoadjuvant Pertuzumab: the Exception That Proves the Rule?
Contrary to some expectations, getting accelerated approval for neoadjuvant therapy does not look easy, and the pertuzumab story may be the exception that proves the rule.
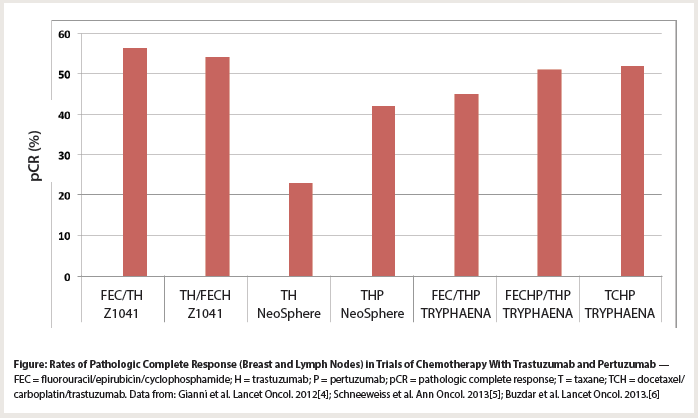
Figure: Rates of Pathologic Complete Response (Breast and Lymph Nodes) in Trials of Chemotherapy With Trastuzumab and Pertuzumab
The original impetus for neoadjuvant therapy for breast cancer was to render patients with locally advanced breast cancer operable. In subsequent clinical trials, the key goals of neoadjuvant treatment were to improve surgical options for patients, and to determine whether earlier administration of systemic therapy would lower the risk of breast cancer recurrence. In the end, that latter objective was not realized-but neoadjuvant therapy nonetheless emerged as a treatment option that could enable less extensive surgery and provide effective “adjuvant” treatment. The identification of pathologic complete response (pCR) as a prognostic marker for outcomes following neoadjuvant chemotherapy made neoadjuvant treatment a favorite model for clinical investigators, since neoadjuvant treatment allowed for rapid ascertainment of clinical activity without the time required for long-term follow-up, made every patient an “event” (permitting smaller study sample sizes), and facilitated extensive correlative studies using serial imaging or tissue biopsies. This enthusiasm led to hundreds of trials of neoadjuvant therapy for breast cancer.
Encouraged by this surge of interest and seeking to move innovative drugs into the market more quickly, the US Food and Drug Administration (FDA) developed a framework for accelerated drug approval based not on traditional measures of benefit to the patient, such as disease-free survival (DFS) or overall survival (OS), but on improvement in the surrogate outcome of rates of pCR.[1] The policy handsomely blended risk and reward considerations for makers of novel drugs. The reward of accelerated approval would be granted based on near-term results with pCR. Full approval would be awarded if long-term follow-up confirmed that there was a DFS or OS benefit to the patient population; the risk was that without such long-term gains, accelerated approval would be withdrawn. The FDA outlined important criteria for studies that might be considered for regulatory intent. Such studies should have an “add-on” design, comparing a standard regimen with the new drug vs without it; they should give all treatment preoperatively, avoiding postoperative therapy or making such treatments uniform; and they should define pCR as pertaining to the breast and lymph nodes. Additional guidance called for placebo-controlled studies and tissue evaluation by pathologists blinded to the treatments. The framework for accelerated approval has been warmly greeted by clinical investigators, advocates, and industry, all hoping to see important new drugs enter the market sooner.
In support of the accelerated approval model, the FDA led a meta-analysis to understand the relationship between improvements in pCR and improvements in DFS or OS. A critical goal of this effort was to define the magnitude of shift in pCR rate that would translate into a long-term DFS benefit. The canonical example was the NOAH trial, which studied chemotherapy ± trastuzumab, and showed a shift in pCR (19% vs 38% with the addition of trastuzumab) and related gain in 3-year DFS (56% vs 71%).[2] The meta-analysis confirmed the prognostic significance of pCR in breast cancer. However, it “could not validate” pCR as a surrogate endpoint for improved outcomes in breast cancer, nor did it identify a threshold for shift or gain in pCR rates that would likely predict benefit, or by analogy, recommend accelerated approval.[3]
The results of the meta-analysis notwithstanding, pertuzumab was granted accelerated approval for use in the neoadjuvant treatment of breast cancer. Interestingly, the studies that led to its approval did not conform to the proposed models for how trials would garner regulatory approval. The NeoSphere study compared nonstandard, taxane-only chemotherapy and trastuzumab, given with or without pertuzumab, and permitted varied treatments out back[4]; it was not an add-on design. Indeed, the FDA label does not recommend administering pertuzumab this way, but instead suggests 6 cycles of pertuzumab-based treatment given with either docetaxel/carboplatin/trastuzumab or anthracyclines/taxanes/trastuzumab. This recommendation was based on the results of the TRYPHAENA study, which compared three different pertuzumab-based regimens but did not ask a question about pertuzumab itself.[5] Neither of these trials was intended for long-term follow-up to confirm the benefits of pertuzumab in the neoadjuvant setting. Those data will come from the APHINITY trial (ClinicalTrials.gov identifier NCT01358877), which employed adjuvant pertuzumab for 52 weeks, far longer than the 3 to 4 months utilized in the neoadjuvant studies. The importance of a “standard” adjuvant chemotherapy/trastuzumab backbone may be critical to the long-term findings on pertuzumab. Neoadjuvant studies such as Z1041, which included standard anthracycline- and taxane-based treatments with trastuzumab, achieved pCR rates similar to those seen with the introduction of pertuzumab in NeoSphere and TRYPHAENA, raising the question of whether pertuzumab will augment long-term results in patients given full chemotherapy and trastuzumab treatments (Figure).[6]
Given these irregularities, how did pertuzumab get approved? Several factors were key. First, as a test of the neoadjuvant approval framework, pertuzumab came “pretty close” to the FDA goals of having neoadjuvant data and the opportunity for long-term follow-up. Second, the drug was already marketed based on a survival difference in the metastatic setting, suggesting that it was more likely to affect the natural history of early-stage disease. Third, and critically, pertuzumab has proven to be extraordinarily safe. In the end, should there be no real clinical advantage with pertuzumab, it is unlikely that patients will have been subjected to short- or long-term harm from neoadjuvant treatment. Thus, a variety of important and pre-existing data and clinical findings were well aligned to support accelerated approval for neoadjuvant pertuzumab.
But that is not typical in drug development. In fact, to reflect on how a variety of data from trials in the metastatic and preoperative settings could be woven together to create a clean story for regulatory approval is also to recognize how unusual a set of circumstances this represents. Drugs with suggestions of benefit in the neoadjuvant setting-such as carboplatin, lapatinib, or bevacizumab-have not been shown to enhance survival in metastastic breast cancer, and come with substantial risks and side effects. Newer drugs with provocative findings in preoperative studies lack data in the metastatic setting. Smaller biotech or pharmaceutical companies with innovative products seeking approval for neoadjuvant indications will have to commit to adequately powered, randomized trials with long-term follow-up to gain full approval-studies that are slower, costlier, and more challenging for accrual than exploratory neoadjuvant trials. Contrary to some expectations, getting accelerated approval for neoadjuvant therapy does not look easy, and the pertuzumab story may be the exception that proves the rule.
Financial Disclosure:The author has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366:2438-41.
2. Gianni L, Eierman W, Semglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377-84.
3. Cortazar P, Zhang L, Untch M, et al. Pathological compete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014. [Epub ahead of print; doi: 10.1016/S0140-6736(13)62422-8]
4. Gianni L, Pienkowski T, Im YN, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 study. Lancet Oncol. 2012;13:25-32.
5. Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early stage breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24;2278-84.
6. Buzdar AU, Suman VJ, Meric-Bernstam F, et al. Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1317-25.