Evolving Strategies for the Management of BRAF-Mutant Metastatic Colorectal Cancer
In this article, we review the characteristics of the BRAF mutation and the evolving strategies for managing BRAF-mutant metastatic colorectal cancer.
Oncology (Williston Park). 33(6):206-11.

Hey Min Lee

Van Morris, MD

Stefania Napolitano, MD

Scott Kopetz, MD, PhD

Figure 1. Kaplan-Meier Survival Curve of BRAF V600E–Mutated mCRC Patients vs BRAF Wild-Type mCRC Patients
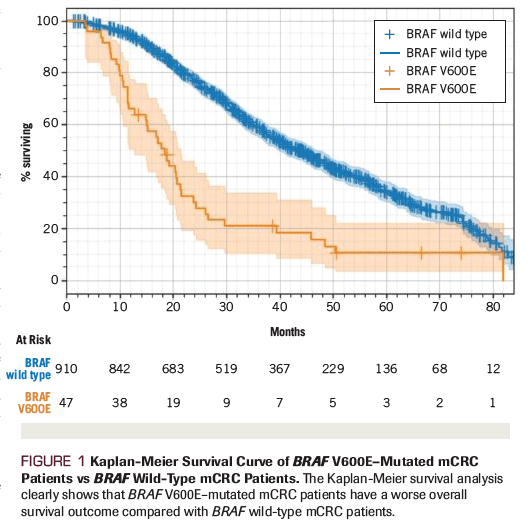
Figure 2. Feedback Activation of EGFR in Response to BRAF Inhibition
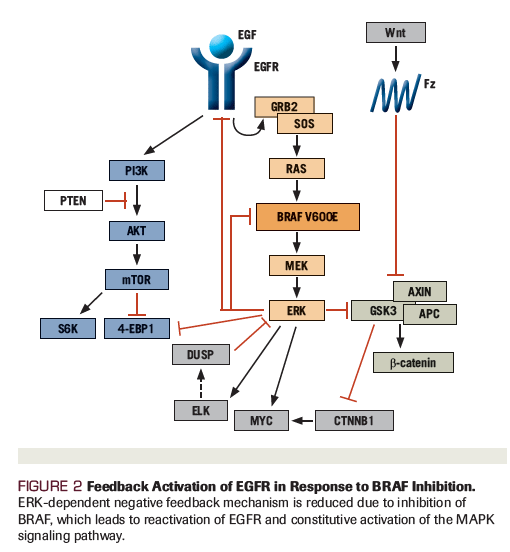
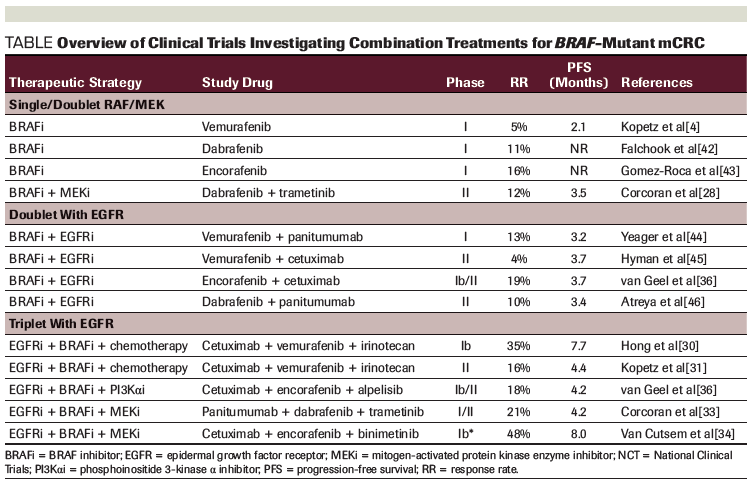
Table. Overview of Clinical Trials Investigating Combination Treatments for BRAF-Mutant mCRC
Detection of the BRAF V600E mutation has important genetic, prognostic, and therapeutic implications for patients with metastatic colorectal cancer (mCRC), as it aids in the identification of a subgroup of patients who derive little benefit from standard treatments and have an extremely poor prognosis. Secondary analyses of BRAF V600E–mutated subsets from multiple randomized clinical trials have demonstrated a lack of therapeutic benefit and poor prognosis with conventional cytotoxic chemotherapy doublets, highlighting the need for novel effective treatments for this subpopulation. In contrast to patients with BRAF V600E–mutated metastatic melanoma, only 5% of patients with BRAF V600E–mutated mCRC responded to BRAF inhibitor monotherapy in an early-phase trial. Basic science efforts to define resistance mechanisms to BRAF inhibition have generated new therapeutic approaches for these patients. As a result, the treatment landscape for mCRC with a BRAF V600E mutation has shifted dramatically in recent years. Promising clinical trials using combination therapies that inhibit the mitogen-activated protein kinase (MAPK) pathway and alternative active pathways have demonstrated major advances for patients with BRAF V600E–mutated mCRC.
Introduction: Clinical and Molecular Characteristics of the BRAF Mutation
Colorectal cancer is the second leading cause of cancer-related death in the United States.[1] It initiates from normal colonic epithelia following multiple genetic and epigenetic events.[2] Approximately 5% to 10% of patients with metastatic colorectal cancer (mCRC) have a mutation of the proto-oncogene BRAF.[3,4] Strong associations between BRAF V600E mutations and female gender, elderly age at diagnosis, and a proximal (right-sided) primary colon tumor location have been found.[5-7]
BRAF mutations are detected in many types of cancer, such as melanoma, colorectal cancer, thyroid cancer, non–small-cell lung cancer, and hairy cell leukemia.[7] In all cancer types, they encode a serine/threonine protein kinase that is associated with the RAS/RAF/MEK/ERK pathway.[7] The majority of BRAF mutations result in an amino acid substitution of valine to glutamic acid within codon 600 (V600E).[5] Compared with BRAF wild-type tumors, this missense BRAF V600E mutation results in downstream phosphorylation of MEK and ERK kinase, leading to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway, which drives tumor cell proliferation and metastasis of colorectal cancer.[7] Less commonly, atypical BRAF mutations are present, which can be characterized as either class II or III subtypes. Class II BRAF mutants signal as constitutive dimers and are RAS independent; while they are resistant to vemurafenib, they may be sensitive to novel RAF dimer inhibitors or MEK inhibitors. Class III BRAF mutants, which lack kinase activity, are associated with activation of RAS by receptor tyrosine kinase signaling, and therefore may be sensitive to epidermal growth factor receptor (EGFR) inhibition in some cases.[8,9]
Tumors with BRAF V600E mutations are often associated with a high mutation burden, microsatellite instability (MSI), and a CpG island methylator phenotype (CIMP), with associated high levels of epigenetic modulation of gene expression through DNA methylation.[3,6,10,11] Furthermore, a recent classification of colorectal cancer, based on intrinsic gene expression profile patterns, described four distinct subgroups called consensus molecular subtypes.[12] BRAF V600E–mutated colorectal cancer tumors are highly linked to consensus molecular subtype 1 (CMS1), and the typical features of this subtype overlap with the molecular characteristics associated with BRAF V600E mutations from the aforementioned analysis conducted by The Cancer Genome Atlas project. As in MSI-high tumors, CMS1 colorectal cancer tumors are also associated with significant immune infiltrations and activation of immune response pathways, as seen by higher populations of type 1 T helper cells, cytotoxic T cells, and natural killer cells.[12,13] Interestingly, BRAF-mutated mCRC tumors emerge in colorectal cancer pathogenesis as a distinct biological entity, often from the sessile serrated adenomatous pathway.[14] The molecular classification of the sessile serrated pathway is distinctly characterized by BRAF mutation, promoter methylation of MLH1, activating mutation in MAPK pathway components, and MSI-high features.[14,15]
Compared with patients with BRAF wild-type mCRC, patients with BRAF V600E–mutated mCRC have an exceptionally poor prognosis and lower response rates to standard therapy. As shown from one series of patients with mCRC assessed at academic and community centers (Figure 1), the median overall survival for patients with BRAF V600E–mutated mCRC was significantly shorter compared with patients with BRAF wild-type mCRC (11 months vs 35 months, respectively).[4,6] Even though BRAF V600E mutations are typically associated with RAS wild-type tumors, several studies have suggested that the BRAF V600E mutation is a predictive marker for limited sensitivity to anti–EGFR monoclonal antibodies (eg, cetuximab, panitumumab) alone or when combined with chemotherapy.[18,19] For example, BRAF V600E–mutant colorectal cancer patients have shown a much lower response rate than patients with BRAF wild-type tumors to cetuximab plus chemotherapy (8.3% vs 38.0%, respectively; P = .0012).[17] Therefore, new therapeutic strategies that are relevant to the unique tumor biology of the BRAF-mutant colorectal cancer population are warranted.
Advances in Treatment for BRAF-Mutated mCRC Patients
Standard cytotoxic combinations of folinic acid, fluorouracil, and oxaliplatin (FOLFOX) and folinic acid, fluorouracil, and irinotecan (FOLFIRI) result in only a modest benefit in the first-line setting; these combinations have limited benefits in the second-line and beyond setting as well, with a median progression-free survival (PFS) of approximately 2 months.[19] Given this limited activity, more aggressive cytotoxic combinations have been evaluated, including fluorouracil, oxaliplatin, and irinotecan (FOLFOXIRI) plus bevacizumab. In a subgroup analysis of 28 patients with BRAF V600E–mutated mCRC treated with FOLFOXIRI plus bevacizumab or FOLFIRI plus bevacizumab, a survival trend was noted for patients receiving FOLFOXIRI plus bevacizumab (hazard ratio [HR], 0.55). However, this finding was not statistically significant, likely due to the small sample size.[20] Despite this statistical limitation, FOLFOXIRI plus bevacizumab is currently considered a potential first-line treatment option for BRAF-mutated patients, assuming that they are able to tolerate this combination.[21]
BRAF inhibitor monotherapy has been tested in BRAF-mutated colorectal cancer and melanoma patients. Dabrafenib, encorafenib, and vemurafenib are oral BRAF inhibitors that have been approved by the US Food and Drug Administration (FDA) for BRAF-mutated metastatic melanoma. The median PFS for these agents as monotherapy for metastatic melanoma patients was 5.3 months,[22] 5.1 months,[23] and 9.6 months,[24,25] respectively. In contrast, response rates to BRAF inhibitors as single agents among patients with BRAF V600E–mutated mCRC have been much lower.[26] For example, a phase II study of 21 patients treated with vemurafenib reported a 5% response rate.[4]
Indeed, suboptimal response to BRAF inhibitor monotherapy is linked to incomplete inhibition of MAPK signaling in colorectal cancer cell lines.[26] Analysis of resistance mechanisms to BRAF-targeted therapies has demonstrated acquired mutations in oncogenes, such as KRAS, NRAS, and MAPK1, as well as copy number amplifications in BRAF, which may be responsible for loss of response.[4,26] Therefore, it is reasonable that additional targeted agents against effectors in this pathway would result in deeper antitumor responses and provide a rationale for dual inhibition of BRAF and MEK as a means to improve response rates in BRAF V600E–mutated mCRC. For BRAF V600E–mutated melanoma, one study reported that the addition of a MEK inhibitor to a BRAF inhibitor resulted in improved response rates as high as 76%, compared with 54% with monotherapy.[27] For BRAF V600E–mutated mCRC patients, response rates to dabrafenib plus trametinib (a MEK inhibitor) have been much lower, with 5 of 43 patients (12%) responding to this combination.[28] Together, these data suggest that reductions in tumors harboring BRAF V600E mutations are dependent on the context of the associated primary tumor, with inferior clinical outcomes noted with targeted therapies against BRAF and MEK for colorectal cancer compared with melanoma.
Overcoming Innate Resistance to Targeted Therapies in BRAF V600E–Mutated mCRC
Preclinical studies using in vitro models of BRAF V600E–mutated colorectal cancer have shown that BRAF inhibition induces reflexive feedback activation of EGFR[28,29] and can propagate constitutive MAPK signaling (Figure 2). Here, EGFR-mediated reactivation of downstream signaling pathways contributes to the innate insensitivity of these tumors to BRAF inhibitor monotherapy. In an early-phase study of patients with BRAF V600E–mutated mCRC testing the combination of vemurafenib together with irinotecan and cetuximab (VIC), 6 of 17 patients (35%) achieved a radiographic response.[30] The median PFS was 7.7 months, longer than the 2 months previously reported in the treatment-refractory population following standard chemotherapy options. A follow-up randomized phase II trial (Southwest Oncology Group 1406) compared irinotecan/cetuximab with or without vemurafenib to test the addition of a BRAF inhibitor to anti-EGFR therapy in BRAF V600E–mutant mCRC patients. The trial demonstrated an improved median PFS of 4.4 months (95% CI, 3.6–5.7 months) compared with 2.0 months (95% CI, 1.8–2.1 months) for patients receiving VIC (HR, 0.42; 95% CI, 0.26–0.66; P < .001), with higher rates of radiographic response and disease control in the experimental arm.[31] Based on the results of this prospective study, the VIC regimen has been added to the National Comprehensive Cancer Network (NCCN) guidelines as a recommended therapy for patients with treatment-refractory BRAF V600E–mutated colorectal cancer.[21]
Triple-combination therapy with MEK and BRAF inhibition plus EGFR-targeted therapies has been tested; one clinical trial evaluated the combination of dabrafenib, panitumumab, and trametinib in patients with BRAF V600E–mutated mCRC.[32] The response rate reported in the expanded phase II study of the triplet therapy was 21% (95% CI, 12.5%–43.3%) compared with 10% in the dabrafenib plus panitumumab arm (95% CI, 1.2%–31.7%).[33] In addition, the ongoing phase III BEACON CRC study is randomizing patients to irinotecan plus cetuximab; encorafenib plus cetuximab; or encorafenib, cetuximab, and binimetinib. Early results from the 29 patients in the safety lead-in portion for encorafenib, cetuximab, and binimetinib showed an impressive overall response rate of 48% (3 complete responses and 11 partial responses), and 15 patients with stable disease, with a PFS of 8 months.[34] Based on the results of this study, the FDA granted a Breakthrough Designation to combination encorafenib, cetuximab, and binimetinib for BRAF V600E–mutated mCRC, as detected by an FDA-approved test, for patients who failed one or two prior lines of therapy for metastatic disease. Finalized mature survival data for this prospective trial are awaited, including the complete results of the phase III trial, but the early signal reported for BRAF plus EGFR plus MEK inhibition in this population appears most promising. An overview of clinical trials of targeted therapies against the MAPK pathway in BRAF V600E–mutated mCRC patients is provided in the Table.
A preclinical study suggests that activation of the phosphoinositide 3-kinase (PI3K)/AKT pathway may serve as as an additional resistance mechanism to BRAF inhibitors, supported by the antitumor activity of dual inhibition of BRAF plus PI3K in BRAF-mutated colorectal cancer cell lines.[35] However, the phase Ib study of encorafenib/cetuximab with or without alpelisib (a PI3Kα inhibitor) in BRAF-mutated mCRC showed no incremental benefit with the addition of alpelisib, with response rates of 19% vs 18% and a median PFS of 3.7 vs 4.2 months for dual- and triple-combination therapies, respectively.[36]
Recommendations for Patient Management
Given the aggressive biology seen in this population, including a rapid decline in performance status in the setting of disease progression, additional practical considerations are warranted. Early testing for BRAF mutations is important to identify optimal treatment and appropriately establish expectations. BRAF mutations are highly concordant between primary and metastatic sites and typically have a high rate of detection in circulating tumor DNA (ctDNA) in plasma, which allows for testing with whichever source material is readily available for a given patient.[30] Our practice is to request BRAF testing in conjunction with NRAS and KRAS testing after the diagnosis of mCRC. We will alternatively utilize ctDNA testing if tissue is not readily available at the facility for next-generation sequencing testing to avoid the delay in procuring outside diagnostic material.
Imaging testing is typically limited to CT scans of the chest, abdomen, and pelvis, which is consistent with other mCRC patients. Patterns of metastases are notable for higher rates of peritoneal disease and retroperitoneal lymph nodes. Anecdotally, atypical sites of metastases are also seen with high rates of bone and brain metastases, although these are not sufficiently common to justify routine testing and instead should be triggered by symptoms or lab abnormalities with a high level of suspicion.
Initial chemotherapy in our practice is FOLFOXIRI plus bevacizumab, with approximately 50% of patients being candidates for such an approach. Progression on first-line therapy can occasionally be subtle in patients with peritoneal-predominant disease, and requires close vigilance to avoid missing opportunities for second-line therapy. Based on the limited activity of second-line cytotoxic chemotherapy, we proceed with targeted therapy with the VIC regimen in the second-line setting. Given the encouraging clinical trial data with alternate strategies, enrollment to clinical trials is encouraged in any line. In our experience, trials should be considered early in the disease course, because it is difficult to predict performance status declines, and at least one study suggested improved outcomes of the same regimen in the second-line vs third-line setting.[34]
The VIC regimen is associated with several characteristic symptoms, including arthralgias and secondary cutaneous malignancies.[31] Arthralgias can be dose-limiting in a minority of patients, but can typically be managed with anti-inflammatory agents instead of dose reductions. Secondary cutaneous malignancies, most commonly keratoacanthomas, can been seen and are usually managed via resection by a dermatologist without requiring dose interruption or discontinuation. Nausea, when present, is manageable, although high-dose 5-hydroxytryptamine 3 receptor (5-HT3) antagonists have the potential to induce QTc prolongation in combination with vemurafenib. Attention to electrolyte abnormalities is encouraged to avoid exacerbating QTc concerns. While intermittent use of low- and moderate-dose 5-HT3 antagonists appears safe, patients are cautioned to avoid continuous dosing and instead alternate with other oral antiemetics. Skin toxicity is similar, if not slightly better, than that seen with cetuximab or panitumumab alone, and is managed by counseling on avoiding sun exposure, and prophylactic use of oral antibiotics and topical corticosteroids. Neutropenia and anemia are also seen with the combination, but are at rates consistent with other combination regimens and do not require special management.
There has been some debate about the utility of aggressive strategies to resect liver-limited disease. Surgical series have reported poor outcomes for several patients, although anecdotal cases showing a benefit have been noted.[37] Since most patients present with more widely disseminated metastatic disease, only a small number of patients with BRAF V600E–mutant tumors are candidates for such surgical strategies. It is our practice to start with chemotherapy for patients with liver-limited disease, but to still consider resection of liver metastases in carefully selected patients.
Not all patients with BRAF V600E–mutant tumors follow the traditional aggressive path, with a minority manifesting with a more traditional or occasionally indolent behavior. As such, personalized management of these patients is required to manage their presenting biology.
Future Research Efforts
Clinical trials on multi-agent combination approaches for the blockade of the MAPK pathway have shown great promise in the treatment of BRAF V600E–mutated mCRC patients. Additional inhibition of ERK, in combination with current targeted therapies, is under investigation. Improvements to targeted therapies could also be useful for treatment of patients with atypical, non–BRAF V600E mutations. However, these mutations are not as common in colorectal cancer, and additional studies are needed to better understand the targetability of them and the associated therapeutic implications.
Other unique molecular characteristics of BRAF-mutated mCRC tumors, such as hypermethylation and immune infiltration, could be potential novel interventions for treatment. For example, epigenetic targeting with DNA methyltransferase (DNMT) inhibitors or histone deacetylase inhibitors in combination with targeted therapy or immunotherapy is under study.[38,39] A preclinical experiment has shown a synergistic effect with the combination of a DNMT inhibitor and vemurafenib in cell lines,[35] which supports further testing of epigenetic modulators in BRAF V600E–mutated mCRC patients.
Another reasonable treatment for BRAF V600E–mutated mCRC patients with concomitant MSI is immune checkpoint blockade with anti–programmed death 1 agents (eg, nivolumab, pembrolizumab) with or without anti–cytotoxic T-lymphocyte–associated antigen 4 agents (eg, ipilimumab). These patients often have high MSI characteristics, which are considered to be predictive markers for a positive response to immunotherapy.[12,40] A phase II study testing nivolumab in patients with MSI-high mCRC found that, among 12 patients with BRAF V600E mutations, an objective response was achieved in 25% (3 patients). The disease control rate (eg, partial response or stable disease lasting at least 12 weeks) was 75% (9 patients).[41] Another phase II trial evaluating nivolumab/ipilimumab for MSI-high mCRC found a 55% objective response rate and a 79% disease control rate in the 29 patients with BRAF V600E–mutated mCRC.[40] Therefore, it appears that patients with BRAF V600E–mutated, MSI-high mCRC can benefit therapeutically from immune checkpoint blockade agents beyond those that target the MAPK signaling pathway.
While BRAF V600E mutations have historically been associated with poor prognoses for colorectal cancer patients, major advances have significantly extended survival outcomes in recent years, backed by corroborating translational sciences. Further understanding of the interplay between the BRAF V600E mutation and associated tumor biology will lead to further treatment advances in the years to come.
Clinical Considerations
• Detection of the BRAF V600E mutation in metastatic colorectal cancer identifies a subgroup of patients who derive little benefit from standard treatments and have an extremely poor prognosis.
• Response rates in BRAF V600E–mutated colorectal cancer patients with BRAF inhibitor monotherapy are lower compared with other BRAF V600E–mutated advanced cancers.
• The addition of targeted therapies against epidermal growth factor receptor and/or MEK to BRAF inhibitors improves outcomes for patients with BRAF V600E–mutated colorectal cancer.
Financial Disclosure:Dr. Morris serves as a consultant to Array Biopharma. Dr. Kopetz receives funding from the National Institutes of Health/National Cancer Institute (grant R01 CA187238); he also serves as a consultant to Amgen, Holy Stone, Merck, and Sympogen. Ms. Lee and Dr. Napolitano have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.

"Colon cancer research has finally joined the club of effective targeted therapy. BRAF, once thought only to be a bad prognostic sign, has now become a real target for therapy. The newest data suggest that hitting the pathway in multiple spots further improves outcomes with acceptable toxicity. Enter a world where BRAF testing is a must for all stage IV CRC."
-John Marshall, MD, Chief, Hematology/Oncology, Medstar Georgetown University Hospital; Director, Otto J. Ruesch Center for the Cure of Gastrointestinal Cancer
References:
1. Ng VY, Scharschmidt TJ, Mayerson JL, Fisher JL. Incidence and survival in sarcoma in the United States: a focus on musculoskeletal lesions. Anticancer Res. 2013;33:2597-604.
2. Zoratto F, Rossi L, Verrico M, et al. Focus on genetic and epigenetic events of colorectal cancer pathogenesis: implications for molecular diagnosis. Tumor Biol. 2014;35:6195-206.
3. Muzny DM, Bainbridge MN, Chang K, et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-7.
4. Kopetz S, Desai J, Chan E, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol. 2015;33:4032-8.
5. Kayhanian H, Goode E, Sclafani F, et al. Treatment and survival outcome of BRAF-mutated metastatic colorectal cancer: a retrospective matched case-control study. Clin Colorectal Cancer. 2018;17:e69-76.
6. Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623-32.
7. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-54.
8. Ikenoue T, Hikiba Y, Kanai F, et al. Advances in brief functional analysis of mutations within the kinase activation segment of B-Raf in human colorectal tumors. Cancer Res. 2003;63:8132-7.
9. Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature. 2017;548:234-8.
10. Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst. 2013;105:1151-6.
11. Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787-93.
12. Guinney J, Dienstmann R, Wang X, De Reyniès A, Schlicker A, Soneson C, et al.: The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350-6.
13. Dienstmann R, Vermeulen L, Guinney J, et al. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. 2017;17:79-92.
14. Tsai JH, Liau JY, Lin YL, et al. Traditional serrated adenoma has two pathways of neoplastic progression that are distinct from the sessile serrated pathway of colorectal carcinogenesis. Mod Pathol. 2014;27:1375-85.
15. Bettington M, Walker N, Clouston A, et al. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367-86.
16. Tsai JH, Liau JY, Lin YL, et al. Traditional serrated adenoma has two pathways of neoplastic progression that are distinct from the sessile serrated pathway of colorectal carcinogenesis. Mod Pathol 2014;27:1375–1385.
17. Zhao B, Wang L, Qiu H, et al. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget. 2017;8:3980-4000.
18. Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705-12.
19. Morris V, Overman MJ, Jiang ZQ, et al. Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clin Colorectal Cancer. 2014;13:164-71.
20. Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-18.
21. Benson AB, Venook AP, Al-Hawary MM, et al. NCCN Guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16:359-69.
22. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-16.
23. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358-65.
24. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315-27.
25. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-19.
26. Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated reactivation of MAPK signaling contributes to insensitivity of BRAF-mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227-35.
27. Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694-1703.
28. Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J Clin Oncol. 2015;33:4023-31.
29. Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100-4.
30. Hong DS, Morris VK, El Osta B, et al. Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAFV600E mutation. Cancer Discov. 2016;6:1352-65.
31. Kopetz S, McDonough SL, Morris VK, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG 1406). J Clin Oncol. 2017;35:520.
32. Bendell JC, Atreya CE, André T, et al. Efficacy and tolerability in an open-label phase I/II study of MEK inhibitor trametinib (T), BRAF inhibitor dabrafenib (D), and anti-EGFR antibody panitumumab (P) in combination in patients (pts) with BRAF V600E mutated colorectal cancer (CRC). J Clin Oncol. 2014;32:3515.
33. Corcoran RB, Andre T, Atreya CE, et al. Research article combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E-mutant colorectal cancer. Cancer Discov. 2018;8:428-43.
34. Van Cutsem E, Cuyle P, Huijberts S, et al. O-027 BEACON CRC study safety lead-in: assessment of the BRAF inhibitor encorafenib + MEK inhibitor binimetinib + anti–epidermal growth factor receptor antibody cetuximab for BRAFV600E metastatic colorectal cancer. Ann Oncol. 2018;29:mdy149.026.
35. Mao M, Tian F, Mariadason JM, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res. 2013;19:657-67.
36. Van Geel RMJM, Tabernero J, Elez E, et al. A phase Ib dose-escalation study of encorafenib and cetuximab with or without alpelisib in metastatic BRAF-mutant colorectal cancer. Cancer Discov. 2017;7:610-9.
37. Yamashita S, Chun YS, Kopetz SE, Vauthey JN. Biomarkers in colorectal liver metastases. Br J Surg. 2018;105:618-27.
38. Carter CA, Oronsky BT, Roswarski J, et al. No patient left behind: The promise of immune priming with epigenetic agents. Oncoimmunology. 2017;6:1-13.
39. Carson R, Celtikci B, Fenning C, et al. HDAC inhibition overcomes acute resistance to MEK inhibition in BRAF-mutant colorectal cancer by downregulation of c-FLIPL. Clin Cancer Res. 2015;21:3230-40.
40. Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773-9.
41. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182-91.
42. Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893-901.
43. Gomez-Roca CA, Delord J, Robert C, et al. Encorafenib (LGX818), an oral BRAF inhibitor, in patients (pts) with BRAF V600E metastatic colorectal cancer (mCRC): Results of dose expansion in an open-label, phase 1 study. Ann Oncol. 2014;25(suppl 4):iv182-3.
44. Rona D. Yaeger , Andrea Cercek , Eileen Mary O’Reilly , et al. Pilot study of vemurafenib and panitumumab combination therapy in patients with BRAF V600E mutated metastatic colorectal cancer. J Clin Oncol. 2015;33(suppl):abstr 611.
45. Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726-36.
46. Atreya CE, Van Cutsem E, Bendell JC, et al. Updated efficacy of the MEK inhibitor trametinib (T), BRAF inhibitor dabrafenib (D), and anti-EGFR antibody panitumumab (P) in patients (pts) with BRAF V600E mutated (BRAFm) metastatic colorectal cancer (mCRC). J Clin Oncol. 2015;33(suppl):abstr 103.
