Suicide in Patients With Cancer: Identifying the Risk Factors
This article reviews the prevalence of suicide in patients with cancer, risk factors, related conditions, and interventions to identify and treat suicidal patients.
Oncology (Williston Park). 33(6):221-6.

Daniel C. McFarland, DO

Leah Walsh, MS

Stephanie Napolitano, MA

Jody Morita, MD

Reena Jaiswal, MD

Table. Suicide Risk and Protective Factors in Cancer
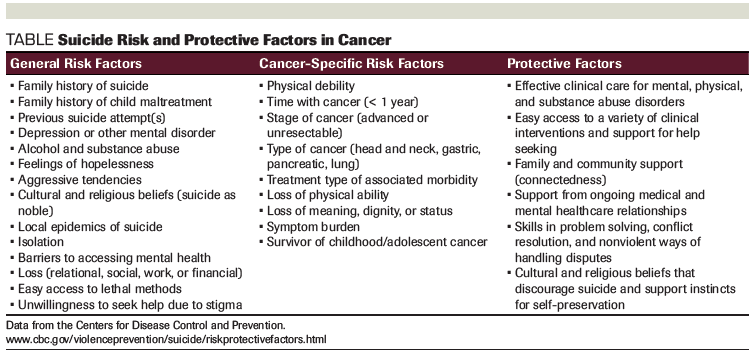
Thoughts of suicide while dealing with cancer are exceedingly common, though relatively few patients make a suicide attempt or complete suicide. Suicide rates among cancer patients are generally thought to be twice as high as that of the general population. However, patients with certain cancer types are at much higher risk for suicide; patients may also be more at risk at certain times during their cancer trajectory. While it is not possible to predict a suicidal act, key features identify those who should be screened more closely. Depression, psychiatric history, previous suicide attempts, hopelessness, demoralization, pain, lack of social support, feeling like a burden to others, and existential concerns (regret, loss of meaning, purpose, and dignity), along with specific demographic characteristics and cancer types confer increased suicidality. Oncologists play a crucial role in identifying these high-risk patients. The Columbia-Suicide Severity Rating Scale is a well-established screening instrument that staff members can use to assess suicidal thinking in patients.
Introduction
A diagnosis of cancer invariably brings thoughts of mortality to the forefront of patients’ minds and may be associated with stigma, social isolation, and personal stressors. While there are many risk factors associated with suicide in both cancer and non-cancer settings, the primary issue for patients with cancer is that the disease leads to physical, mental, and spiritual strains on personal resources. These factors overlay predispositional personal characteristics and may lead to depression and suicide. The actual rate of suicide in cancer patient populations is likely underreported due to “accidental” or unintentional deaths resulting from medication mismanagement, for example. Suicidal thinking exists on a spectrum, from transient passive thoughts about suicide, to active thinking, ruminating, planning, enacting (gesturing), and completing suicide. The key to suicide prevention is identification and proactive management of patients in the oncology setting with a team approach. This article will review the prevalence of suicide in patients with cancer, risk factors, related conditions (eg, suicidal ideation, desire for hastened death), and interventions to identify and treat suicidal patients.
Incidence of Suicide in Cancer Patients
Many people who complete suicide have chronic medical conditions. While rates of suicide are higher in patients with cancer compared with the general population, the speculative reasons for an association between cancer and suicide are particularly intriguing. Shneidman conceptualized the core driving characteristic of suicide as psychological pain, which he called “psychache.”[1] In this way, all affective states (rage, hostility, depression, shame, guilt, hopelessness) are relevant to suicide, since they are psychologically painful. Without psychache, there is no suicide. Others have theorized interpersonal loss and the desire to join the lost person as drivers of suicide.[2] Family members and other survivors of a suicide feel confused after the suicide has occurred. It can be extremely difficult to understand why a person completes suicide. Theories and risk factors can help gain some understanding.
The cancer experience is multitudinously painful for patients and their families. Each specific cancer type leads to various types of morbidity and, consequently, different types of loss. This is reflected in suicide prevalence rates among different cancer types. Although the relative risk of suicide for cancer patients is approximately twice that of the general population, cancer types with a higher rate of physical morbidity, such as head and neck, lung, and pancreatic cancer, have significantly higher rates of suicide.[3] Although suicide is the second leading cause of death among 15- to 29-year-olds, rates of suicide increase with age in general.[4] Since cancer disproportionally affects older individuals, these two entities may combine to affect suicide risk in cancer patients. While there are gender differences in suicide attempts and completions in the general population, there does not appear to be a significant gender difference in the cancer patient population.[5]
Suicide is a permanent solution for a temporary problem-the psychache-that can be addressed and ameliorated. A surprisingly high percentage of cancer patients think about suicide; yet, in terms of absolute numbers, few will make an attempt or complete suicide.[6] Addressing suicide means facilitating prevention through identification of high-risk individuals, as well as treatment of psychache or physical, spiritual, and emotional suffering.
Risk Factors
Suicide risk factors for patients with cancer include both cancer-related and general suicide risk factors (Table). Risk factors may interact with each other to create a higher risk. Cancer as a chronic disease is a risk factor for suicide, as are other comorbid chronic medical conditions. Demographic and disease-related factors are associated with increased risk for suicide among patients with cancer, as well as the general population.[7] Understanding these risk factors can guide medical professionals in discussing a patient’s motivation to commit suicide. Patients with particular demographic characteristics, including gender, age, and marital status, can be at an increased risk for committing suicide. Male patients with cancer generally tend to have higher rates of suicide compared with the general population.[8] When compared across cancer patients, men with cancer are at highest risk for suicide, particularly if they are older, white, and unmarried.[9] Older patients and patients who are not married may be at increased risk for suicide generally, especially older white men; however, other research suggests that at the time of diagnosis, younger patients are at the highest risk for suicide compared with any other age.[8,9]
Specific aspects of patients’ diagnoses can put them at an increased risk for suicide, such as cancer type, stage at diagnosis, length of time since diagnosis, and cancer-related symptoms (eg, pain). Symptoms related to both cancer and its treatment further augment suicide risk. While individual studies have found particular cancer types to be associated with suicide risk, a review of the literature shows a lack of consensus and posits that illness severity and progression are most closely linked to suicide risk compared with disease type.[10] Patients experiencing pain, fatigue, and general physical dysfunction are at a higher risk for suicide.[11] For example, patients with cancers diagnosed at advanced stages or that are unresectable such as lung or pancreatic cancer seem to be at highest risk for committing suicide.[8,9] The cancer types with the highest rates of suicide are lung, head and neck, gastric, and pancreatic cancer.[4,12]
Of particular importance for the clinician assessing suicide risk, patients with cancer are most likely to commit suicide within the first year of diagnosis.[13,14] The relative risk is highest immediately after or during the first week after diagnosis, incurring a relative risk of 12.6 (95% CI, 8.6–17.8), which goes down to a relative risk of 4.8 during the first 3 months after diagnosis. Additionally, the risk is three times more likely during the first year after diagnosis and two times more likely after 1 year.[15] Cancer-related suicide risk generally decreases with time, but remains elevated for the first 5 years after diagnosis.[16,17] On the contrary, patients with certain cancers (eg, bladder and renal cancer) may still have a high risk of suicide over time.[18] In addition to length of time since diagnosis, the stage of disease at diagnosis is related to suicidality, such that patients with more advanced diseases at diagnosis are at higher risk for suicide.[3]
Demographic and clinical risk factors for suicide within specific cancer types have been examined and appear to be cumulative. For example, patients with non–small-cell lung cancer who are male, white, unmarried, and between the ages of 60 to 75 years at diagnosis are at higher risk for suicide than other patients with this specific diagnosis.[19] Interestingly, patients who underwent surgery for their cancer were also at a higher risk for suicide than those who did not undergo surgery.[20] In the context of prostate cancer, even when matched on demographic characteristics, including age and geographic region, elderly men had over a four-fold increase in suicide risk compared with the general population.[21] This may be due to the psychological pain related to impotence or sexual dysfunction.
These augmented risks highlight the cumulative distress and challenges faced by patients with cancer, which may lead to suicide. In contradistinction to decreasing suicide risk over time, patients with bladder and kidney cancer pose the highest risk for committing suicide among the genitourinary cancers, and this risk increases as time since diagnosis increases.[18] Alternatively, in a study by Kam and colleagues using Surveillance, Epidemiology, and End Results (SEER) data, it was noted that thyroid cancer was the only head and neck cancer without an elevated suicide risk, likely due to the high prevalence of localized resectable disease.[17] Head and neck cancer patients who received specific combinations of treatment, specifically those who completed radiation therapy but did not undergo surgery, had the highest rates of suicide. Treatment type and its side effects and sequelae leave a lasting psychological impact that contributes to higher risk of suicide.
Adult survivors of childhood cancers as a group are particularly vulnerable to suicide and should be monitored closely.[22] While epidemiologic risk may identify high-risk patients, any patient may complete suicide; therefore, clinical “red flags” are likely the most worthwhile identification tool that clinicians have to prevent suicide.
Suicidal Ideation
Suicidal ideation and thinking about suicide is predictive of suicide.[23] Suicidal ideation and history of suicidal behavior are among the most salient short- and long-term risk factors for suicide.[24,25] While there is usually a prodromal period of suicidal ideation (presuicidal syndrome), which can vary from transient and passive to ruminative and disruptive and lead to suicidal planning or enactment, suicide can occur impulsively without immediate warning. That is to say, suicidal ideation and actual suicide do not always occur together.[26] It is very common for anyone facing mortality via a chronic medical illness, especially those causing significant morbidity (eg, neurologic illness), to contemplate death and even suicide in the form of “maybe X would be better off without me.” These thoughts are related to a life adjustment, and should be transient and not overtly bothersome or persistent. When suicidal ideation becomes persistent or ruminative, patients may begin to feel unsafe. Chochinov et al found that 44.5% of 200 terminally ill patients reported a fleeting desire for death, while 8.5% had a more enduring wish for death.[27] There is always ambivalence, negotiation, and equivocation about suicide and, with this uncertainty, mental health treatment can effectively intervene. Patients may begin having magical thinking or looking for signs to help them decide whether or not to commit suicide. At a further level of suicidality, patients may have formulated a plan that could be enacted either impulsively (eg, due to uncontrolled emotion, lack of ability to regulate emotion, or facing disappointment) or in a deliberately controlled way. Patients often enact a suicidal gesture that is not actually meant to complete suicide but represents internal negation or preparation. Unfortunately, these acts are often unintentionally fatal. Even at this point, few patients are truly sure of their decision and will often reach out through subtle gestures. These patients can be helped greatly by an astute clinician who notices changes in cognition, emotion, and personality.
Desire for Hastened Death
Another aspect of suicidal thinking in patients with cancer is the psychological construct known as the desire for hastened death (DHD), or the longing for death to occur more rapidly than it otherwise would.[28] DHD incidence varies but increases while approaching the end of life and is more common in palliative and hospice settings.[29] The most common reasons noted for DHD include depression, hopelessness, perception of being a burden to others, and forfeiture of personal independence.[30] Disease symptoms were also included amongst the most cited factors, along with fear of pain and other anticipated suffering. Similar to suicidal ideation, depression and hopelessness have been found to be independent factors related to DHD.[28] There are several validated measures for assessing DHD in a clinical setting, including the most commonly used Schedule of Attitudes toward Hastened Death and the Desire for Death Rating Scale.[27,31]
The optimal approach to addressing DHD is unclear. DHD is highly correlated with depression; therefore, addressing underlying depression is an appropriate approach. It has been suggested that DHD responds to psychological support specifically over other forms of comfort care.[32] For instance, pain management does not necessarily correspond to a decrease in DHD.[33] Therefore, it is essential that clinicians provide adequate psychological support.
Physician-Assisted Suicide (PAS)
PAS is legal in California, Colorado, Hawaii, Montana, Oregon, Vermont, and Washington, as of January 1, 2019. Death with dignity statues allow adult state residents with a confirmed prognosis of 6 or fewer months to live to hasten their inevitable or imminent death. States vary on procedures to implement PAS, but frequently require more than one physician to agree, as well as mental health evaluations and waiting periods.[34] While aid-in-dying laws are meant to protect patient autonomy, the act is problematic for physician beneficence or nonmaleficence and remains controversial, with opposition from some medical societies.[35]
Clinical Factors
Depression
The vast majority of suicides are associated with ongoing depression and underlying psychiatric history. Depression is the most common mental illness associated with suicide, which has been documented in 75% of cancer-related suicides.[36] Depression incurs a 25-fold increased risk of suicide compared with those without depression, irrespective of cancer.[37,38] Depression is most often the strongest predictor of suicide or DHD and is therefore a logical treatment target for preventing suicide. Pathological depression is often difficult to differentiate from appropriate feelings of sadness among patients facing a terminal illness.
Hopelessness
Hopelessness is a separate construct from depression and is a unique risk factor for both DHD and suicidal ideation.[39,40] Hopelessness in combination with depression puts patients at particularly high risk for developing suicidal ideation.[41]
Demoralization
Demoralization is similar to hopelessness but denotes a perceived inability to cope and is associated with a loss of meaning and a sense of disheartenment. It is quite common in cancer settings and may be a stronger predictor for suicidal ideation than depression.[6] Additionally, it may occur independently of depression.
Pain
While pain leads to heightened psychological distress and depression, the relationship with suicide is actually more complex. One study found that pain was not associated with suicide,[42] and other studies have suggested that the physical limitations that pain creates are responsible for suicidal ideation and DHD.[43]
Social support
A lack of social support is associated with DHD, suicidality, and requests for euthanasia.[44] Relationships heavily influence suicidal thinking. Restoration of relationships or a corrective interpersonal experience to take the place of actual or perceived interpersonal losses can be healing and protective for many patients.
Burden to others
While 19% to 65% of terminally ill patients express feelings of being a burden to others, a retrospective study found that the feeling was universal among those who completed suicide.[45,46]
Personality traits
Patients who are accepting and adaptable are less likely to contemplate suicide; however, patients with concerns about loss of autonomy, dependency, and a strong need to control circumstances are more likely to have suicidal ideation.[47]
Psychiatric history
Patients with a previous psychiatric disturbance and treatment are more likely to contemplate suicide.[42] In fact, one could argue that it is quite rare for someone without a psychiatric history of some kind to attempt or complete suicide. Many psychiatric conditions are concomitantly associated with depression (eg, bipolar depression, schizoaffective disorder, substance abuse disorders, personality disorders, along with all of the subsyndromal conditions such as adjustment disorders and minor depressive states), which places most patients with psychiatric disorders at higher risk of suicide.
Existential concerns
Patients confronted with mortality often struggle with loss of meaning, purpose, and dignity; regret; and an understanding of what happens after death, all of which may lead to suicidal thinking. Reports of low spiritual well-being or loss of dignity are associated with suicidal thinking.[44,48]
Interventions
Suicidal thinking is a deliberation between the will to live or die.[49] Intervening to reduce suffering, restore connectedness, and maintain safety defuses suicidal thinking. Oncologists are frequently first responders to cancer patients’ psychological suffering. A strong alliance between patient and oncologist can be protective against suicide.[50] A study of young cancer patients found that therapeutic alliance was associated with less suicidal thinking, even after controlling for well-established predictors of suicidal ideation, social supports, and mental health utilization.[51] This study speaks to the unique relationship between oncologists and their patients and the multidimensional nature of therapeutic alliance. Active listening, concern for patients’ well-being, openness, and providing clear explanations benefit patients beyond providing good oncologic care. Oncologists play a critical role, since many patients refuse to see mental health clinicians. Various studies have shown that communication skills training can improve oncologist–patient communication.[52]
There is a need to normalize conversations about suicide in order to get appropriate resources to the right patients. These conversations can be difficult, but asking and talking about suicide can prevent it even when done by non-professionals.[53] The Columbia-Suicide Severity Rating Scale (C-SSRS) is a suicide screening measure that has been validated in multiple settings and has numerous studies supporting its psychometric validity.[54,55] It can be used by non–mental health clinicians and non-clinicians with reliability.[56] Other suicide assessment tools include the National Institute of Mental Health Ask Suicide-Screening Questions (ASQ) Toolkit and the Distress Assessment and Response Tool (DART), which was developed specifically for cancer patients.[57] Screening instruments should assess for any “non-zero” intent to die, since suicidal ideation generally fluctuates and motives may be mixed.[58]
In addition, concomitant mental health services should be made available to patients with cancer who are suffering psychologically. When suicidal thinking is in question, patients should not be started on antidepressant medications without psychological follow-up to assess ongoing cognitions. Distress screening initiatives are designed to triage patients in a step-wise manner to appropriate levels of psychological support.[59] Unfortunately, these services are not always available; efforts to extend psychiatric services through telepsychiatry and collaborative care models are being developed and implemented.[60]
When a Patient Completes Suicide
Almost half of patients who die by suicide are seen by a healthcare provider the month prior to their death.[61] Oncologists may be the last point of contact in the healthcare system for many cancer patients who complete suicide. For the healthcare team, the aftermath of a patient suicide can be a particularly difficult and trying emotional experience that lingers, potentially leading to burnout and compromising the provision of subsequent patient care. “Postvention,” a term coined by the suicidologist Edwin Shneidman, is a series of interventions to support the bereaved after a suicide. The objectives are to provide care to bereaved survivors, caregivers, and healthcare providers; destigmatize the tragedy of suicide; operationalize the confusing aftermath; assist with the recovery process; and act to prevent further suicides by providing support services to survivors.[62] The oncologist may want to assure that mental health care options are offered and provided for staff and for bereaved family members.
Upon initially learning that a patient has completed suicide, oncologists may feel a sense of shock or disbelief. This dissociation from intense feelings-a reaction often reinforced by medical training-can serve a protective function, mitigating the interference caused by grief with a physician’s ability to practice medicine. It is not uncommon to feel a sense of guilt or shame related to the fear that something was missed.[63] These feelings are understandable, but it is important to recognize that the accuracy of suicide prediction models is near zero, even though we can identify patients who may be at an elevated risk for suicide.[64] There is no “one size fits all” model, which is similar to coming to terms with any traumatic event. Many clinicians find it useful to discuss the situation with colleagues and to hear others’ experiences of losing a patient to suicide.[65] This can decrease the sense of isolation that often results from a patient suicide.
Conclusion
Thoughts of suicide in patients with cancer are compounded by chronic disease, advancing age, and multiple losses and reasons for suffering (ie, psychache). Suicide in cancer patients occurs mostly in elderly age groups; older, white, unmarried men are historically most at risk, but patients with head and neck, lung, pancreatic, and stomach cancers are also particularly at risk. Suicidal thinking occurs on a spectrum and presents as suicidal ideation or DHD. In addition, suicide may now be completed legally in certain states with PAS. Depression, hopelessness, demoralization, pain, lack of social support, feeling like a burden to others, a strong need to control circumstances, and existential concerns can all be associated with suicide. Oncologists play an integral role in identifying high-risk patients and providing support. The C-SSRS is a well-accepted and reliable suicide assessment tool; suicide screening should categorize any non-zero intent to die as suicidal ideation. Additionally, it is important to acknowledge the emotional toll that patient suicides have on clinicians and the healthcare team; psychological support should be provided in these situations.
Financial Disclosure:The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
PERSPECTIVE

More Resources Than Ever, but Suicide Risk in Patients and Providers Hasn’t Changed
Nora Janjan, MD, MPSA, MBA
Consistent with the American Medical Association Code of Medical Ethics, the predicate for this article is that healthcare professionals should prevent suicide by also treating the psychological and physical symptoms that cause the “desire for hastened death.” Along with the factors that contribute to suicidal ideation, McFarland and colleagues acknowledge legal constructs in their article, such as physician-assisted suicide and aid-in-dying laws that now exist in the United States. An added burden is on physicians, who themselves are at increased risk for suicide. In fact, this was the topic of McFarland’s talk at the 2019 American Society of Clinical Oncology Annual Meeting earlier this month, “Addressing Depression, Burnout, and Suicide in Oncology Physicians.”
Between 1998 and 2017, more than 4,200 prescriptions were written for physician-assisted suicide drugs, and 63% of these patients chose to end their lives. Of those prescribed these agents, 51% were male, 94% were Caucasian, 48% were college educated, and most were 69 to 89 years old; more than half (63%) had cancer, ranging from 54% in California to 83% in Vermont.[1] Ironically, during the period of time that individual states were passing physician-assisted suicide and aid-in-dying laws, many major shifts in American culture and therapeutic approaches occurred. The populations of the seven states that legalized physician-assisted suicide constitute almost 20% of the total US population.
During this time, marijuana has been legalized in 20 states; while 10 states have fully legalized its use for all purposes, an additional 10 legalized its use for medical reasons. A decade ago, the Affordable Care Act was enacted, which was intended to provide insurance to the uninsured, and to overcome fears of medical bankruptcy through regulatory requirements within insurance plans. Underscoring core principals of medical ethics, pain control and symptom management became important components of healthcare, especially for cancer patients. Social media has also burgeoned, allowing for personal connectivity despite schedules and distance.
Despite all of these changes, the question remains: why do cancer patients still have unmet needs and contemplate or choose suicide? Why do well-paid oncologists need to work an average of 63 hours per week, suffering from burnout at a rate of 25% to 36%, which sometimes also leads to suicide?[2,3] The fact remains that more than 300 physicians die by suicide in the United States each year. Great strides have been made in the culture of cancer care, and yet, suicide persists, among both patients and their providers. Perhaps many of the described symptoms of “psychache,” or suffering, also reflect an existential change in American culture.
Financial Disclosure:Dr. Janjan has no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References
1. ProCon.org. State-by-state physician-assisted suicide statistics. https://euthanasia.procon.org/view.resource.php?resourceID=006992. Accessed May 22, 2019.
2. Banerjee S, Califano R, Corral J, et al. Professional burnout in European young oncologists: results of the European Society for Medical Oncology (ESMO) Young Oncologists Committee Burnout Survey. Ann Oncol. 2017;28:1590-6
3. Murali K, Banerjee S. Burnout in oncologists is a serious issue: what can we do about it? Cancer Treat Rev. 2018;68:55-61.
Dr. Janjan is Senior Fellow at the National Center for Policy Analysis, Dallas, Texas, and an Adjunct Professor in the Department of Environmental and Occupational Health in the School of Public Health at Texas A&M University, College Station, Texas. She is also an Editor-in-Chief of ONCOLOGY.
References:
1. Leenaars AA. Lives and deaths: biographical notes on selections from the works of Edwin S. Shneidman. Suicide Life Threat Behav. 2010;40:476-91.
2. Van Orden K, Witte T, Cukrowicz K, et al. The interpersonal theory of suicide. Psychol Rev. 2010;117:575-600.
3. Misono S, Weiss NS, Fann JR, et al. Incidence of suicide in persons with cancer. J Clin Oncol. 2008;26:4731-8.
4. Miller M, Mogun H, Azrael D, et al. Cancer and the risk of suicide in older Americans. J Clin Oncol. 2008;26:4720-4.
5. Akechi T, Okamura H, Nakano T, et al. Gender differences in factors associated with suicidal ideation in major depression among cancer patients. Psychooncology. 2010;19:384-9.
6. Vehling S, Kissane DW, Lo C, et al. The association of demoralization with mental disorders and suicidal ideation in patients with cancer. Cancer. 2017;123:3394-401.
7. Rosenstein DL. Depression and end-of-life care for patients with cancer. Dialogues Clin Neurosci. 2011;13:101-8.
9. Zaorsky NG, Zhang Y, Tuanquin L, et al. Suicide among cancer patients. Nat Commun. 2019;10:207.
10. Robson A, Scrutton F, Wilkinson L, MacLeod F. The risk of suicide in cancer patients: a review of the literature. Psychooncology. 2010;19:1250-8.
11. Hughes MK. Suicide screening in the oncology population. J Adv Pract Oncol. 2016;7:101-4.7.
12. Robinson D, Renshaw C, Okello C, et al. Suicide in cancer patients in South East England from 1996 to 2005: a population-based study. Br J Cancer. 2009;101:198-201.
13. Ahn MH, Park S, Lee HB, et al. Suicide in cancer patients within the first year of diagnosis. Psychooncology. 2015;24:601-7.
14. Saad AM, Gad MM, Al-Husseini MJ, et al. Suicidal death within a year of a cancer diagnosis: a population-based study. Cancer. 2019;125:972-9.
15. Fang F, Keating NL, Mucci LA, et al. Immediate risk of suicide and cardiovascular death after a prostate cancer diagnosis: cohort study in the United States. J Natl Cancer Inst. 2010;102:307-14.
16. Hem E, Loge JH, Haldorsen T, Ekeberg O. Suicide risk in cancer patients from 1960 to 1999. J Clin Oncol. 2004;22:4209-16.
17. Kam D, Salib A, Gorgy G, et al. Incidence of suicide in patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2015;141:1075-81.
18. Klaassen Z, Jen RP, DiBianco JM, et al. Factors associated with suicide in patients with genitourinary malignancies. Cancer. 2015;121:1864-72.
19. Zhou H, Xian W, Zhang Y, et al. Trends in incidence and associated risk factors of suicide mortality in patients with non-small cell lung cancer. Cancer Med. 2018;7:4146-55.
20. Osazuwa-Peters N, Simpson MC, Zhao L, et al. Suicide risk among cancer survivors: head and neck versus other cancers. Cancer. 2018;124:4072-9.
21. Llorente MD, Burke M, Gregory GR, et al. Prostate cancer: a significant risk factor for late-life suicide. Am J Geriatr Psychiatry. 2005;13:195-201.
22. Recklitis CJ, Diller LR, Li X, et al. Suicide ideation in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28:655-61.
23. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry. 1999;56:617-26.
24. Beck AT, Brown GK, Steer RA, et al. Suicide ideation at its worst point: a predictor of eventual suicide in psychiatric outpatients. Suicide Life Threat Behav. 1999;29:1-9.
25. Brown GK, Beck AT, Steer RA, Grisham JR. Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol. 2000;68:371-7.
26. Fawcett J. Suicide risk factors in depressive disorders and in panic disorder. J Clin Psychiatry. 1992;53(suppl):9-13.
27. Chochinov HM, Wilson KG, Enns M, et al. Desire for death in the terminally ill. Am J Psychiatry. 1995;152:1185-91.
28. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284:2907-11.
29. Bellido-Pérez M, Monforte-Royo C, Tomás-Sábado J, et al. Assessment of the wish to hasten death in patients with advanced disease: a systematic review of measurement instruments. Palliat Med. 2017;31:510-25.
30. Hudson PL, Kristjanson LJ, Ashby M, et al. Desire for hastened death in patients with advanced disease and the evidence base of clinical guidelines: a systematic review. Palliat Med. 2006;20:693-701.
31. Rosenfeld B, Breitbart W, Galietta M, et al. The schedule of attitudes toward hastened death: measuring desire for death in terminally ill cancer patients. Cancer. 2000;88:2868-75.
32. Ransom S, Sacco WP, Weitzner MA, et al. Interpersonal factors predict increased desire for hastened death in late-stage cancer patients. Ann Behav Med. 2006;31:63-9.
33. O’Mahony S, Goulet J, Kornblith A, et al. Desire for hastened death, cancer pain and depression: report of a longitudinal observational study. J Pain Symptom Manage. 2005;29:446-57.
34. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy. Physician-Assisted Death: Scanning the Landscape: Proceedings of a Workshop. Washington (DC): National Academies Press (US); 2017 Jun.
35. Snyder Sulmasy L, Mueller PS, Ethics, Professionalism, and Human Rights Committee of the American College of Physicians. Ethics and the legalization of physician-assisted suicide: an American College of Physicians position paper. Ann Intern Med. 2017;167:576-8.
36. de la Grandmaison GL, Watier L, Cavard S, Charlier P. Are suicide rates higher in the cancer population? An investigation using forensic autopsy data. Med Hypotheses. 2014;82:16-9.
37. Guze SB, Robins E. Suicide and primary affective disorders. Br J Psychiatry. 1970;117:437-8.
38. Robins E, Murphy GE, Wilkinson RH Jr, et al. Some clinical considerations in the prevention of suicide based on a study of 134 successful suicides. Am J Public Health Nations Health. 1959;49:888-99.
39. Beck AT, Kovacs M, Weissman A. Hopelessness and suicidal behavior: an overview. JAMA. 1975;234:1146-9.
40. Minkoff K, Bergman E, Beck AT, Beck R. Hopelessness, depression, and attempted suicide. Am J Psychiatry. 1973;130:455-9.
41. Chochinov HM, Wilson KG, Enns M, Lander S. Depression, hopelessness, and suicidal ideation in the terminally ill. Psychosomatics. 1998;39:366-70.
42. Rosenfeld B, Breitbart W, Gibson C, et al. Desire for hastened death among patients with advanced AIDS. Psychosomatics. 2006;47:504-12.
43. Filiberti A, Ripamonti C, Totis A, et al. Characteristics of terminal cancer patients who committed suicide during a home palliative care program. J Pain Symptom Manage. 2001;22:544-53.
44. Chochinov HM, Hack T, Hassard T, et al. Understanding the will to live in patients nearing death. Psychosomatics. 2005;46:7-10.
45. McPherson CJ, Wilson KG, Murray MA. Feeling like a burden to others: a systematic review focusing on the end of life. Palliat Med. 2007;21:115-28.
46. Sullivan AD, Hedberg K, Hopkins D. Legalized physician-assisted suicide in Oregon, 1998-2000. N Engl J Med. 2001;344:605-7.
47. Chochinov HM, Kristjanson LJ, Hack TF, et al. Personality, neuroticism, and coping towards the end of life. J Pain Symptom Manage. 2006;32:332-41.
48. McClain CS, Rosenfeld B, Breitbart W. Effect of spiritual well-being on end-of-life despair in terminally-ill cancer patients. Lancet. 2003;361:1603-7.
49. Brown GK, Steer RA, Henriques GR, Beck AT. The internal struggle between the wish to die and the wish to live: a risk factor for suicide. Am J Psychiatry. 2005;162:1977-9.
50. Mack JW, Block SD, Nilsson M, et al. Measuring therapeutic alliance between oncologists and patients with advanced cancer: the Human Connection Scale. Cancer. 2009;115:3302-11.
51. Trevino KM, Abbott CH, Fisch MJ, et al. Patient-oncologist alliance as protection against suicidal ideation in young adults with advanced cancer. Cancer. 2014;120:2272-81.
52. Fallowfield L, Jenkins V, Farewell V, et al. Efficacy of a Cancer Research UK communication skills training model for oncologists: a randomised controlled trial. Lancet. 2002;359:650-6.
53. Lucas MS, Brawner BM, Hardie TL, et al. Assessing suicidal ideation and behaviors among survivors of childhood brain tumors and their mothers during sociobehavioral research. Oncol Nurs Forum. 2015;42:E319-E329.
54. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-77.
55. Arias SA, Zhang Z, Hillerns C, et al. Using structured telephone follow-up assessments to improve suicide-related adverse event detection. Suicide Life Threat Behav. 2014;44:537-47.
56. Kerr DC, Gibson B, Leve LD, Degarmo DS. Young adult follow-up of adolescent girls in juvenile justice using the Columbia Suicide Severity Rating Scale. Suicide Life Threat Behav. 2014;44:113-29.
57. Leung YW, Li M, Devins G, et al. Routine screening for suicidal intention in patients with cancer. Psychooncology. 2013;22:2537-45.
58. Andriessen K. On “intention” in the definition of suicide. Suicide Life Threat Behav. 2006;36:533-8.
59. Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32:1605-19.
60. Sharpe M, Walker J, Holm Hansen C, et al. Integrated collaborative care for comorbid major depression in patients with cancer (SMaRT Oncology-2): a multicentre randomised controlled effectiveness trial. Lancet. 2014;384:1099-108.
61. Ahmedani BK, Simon GE, Stewart C, et al. Health care contacts in the year before suicide death. J Gen Intern Med. 2014;29:870-7.
62. Erlich MD, Rolin SA, Dixon LB, et al. Why we need to enhance suicide postvention: evaluating a survey of psychiatrists’ behaviors after the suicide of a patient. J Nerv Ment Dis. 2017;205:507-11.
63. Gitlin MJ. A psychiatrist’s reaction to a patient’s suicide. Am J Psychiatry. 1999;156:1630-4.
64. Belsher BE, Smolenski DJ, Pruitt LD, et al. Prediction models for suicide attempts and deaths: a systematic review and simulation. JAMA Psychiatry. 2019 Mar 13. [Epub ahead of print]
65. Biermann B. When depression becomes terminal: the impact of patient suicide during residency. J Am Acad Psychoanal Dyn Psychiatry. 2003;31:443-57.

Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.