Immune-Related Adverse Events Involving Multiple Organ Sites in a Patient Treated With Nivolumab Plus Ipilimumab
Experts discuss the case of a 56-year-old white man who presents with multiple immune-related adverse events
Remolina-Bonilla is a clinical researcher at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. Mexico City, Mexico.

Jimenez-Franco is a first-year internal medicine resident at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán.

Lam is an Associate Professor, Department of Medicine, Division of Medical Oncology. University of Colorado Anschutz Medical Campus. Aurora, Colorado.

Bourlon is an Associate Professor, Hematology and Medical Oncology Department. Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. Mexico City, Mexico.

FIGURE 1. Rash Before (A) and After a Systemic Steroid Course (B)
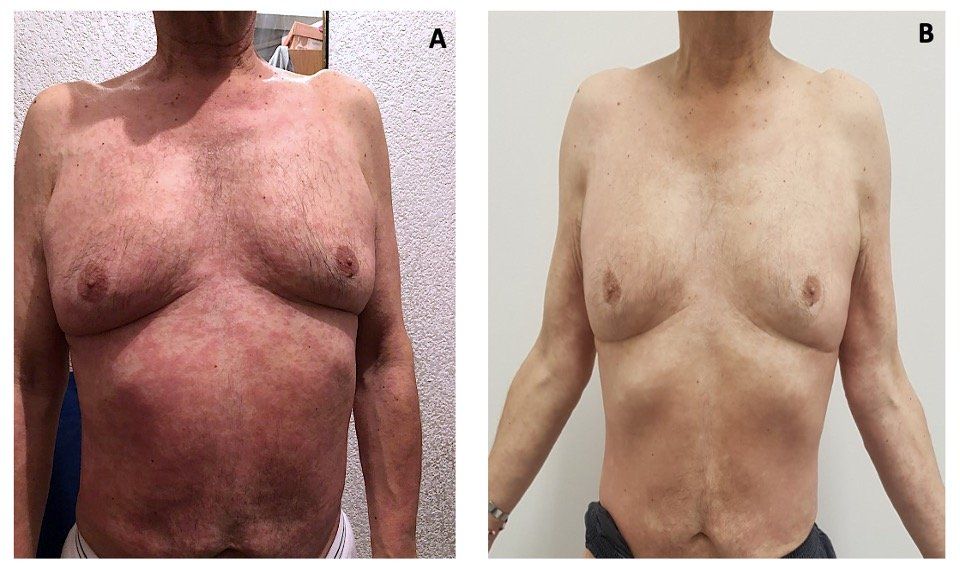
Figure 2. Evolution of Serum Creatinine Values
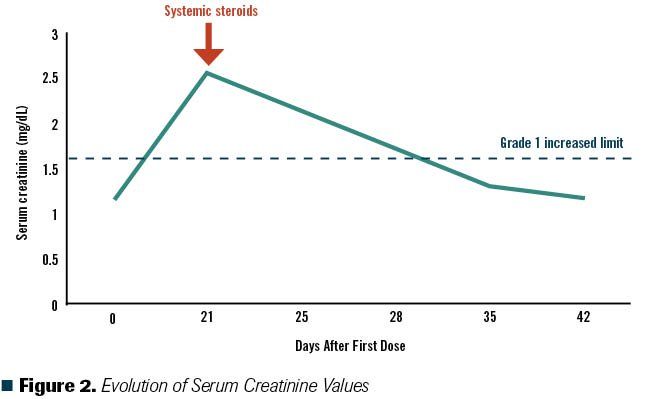
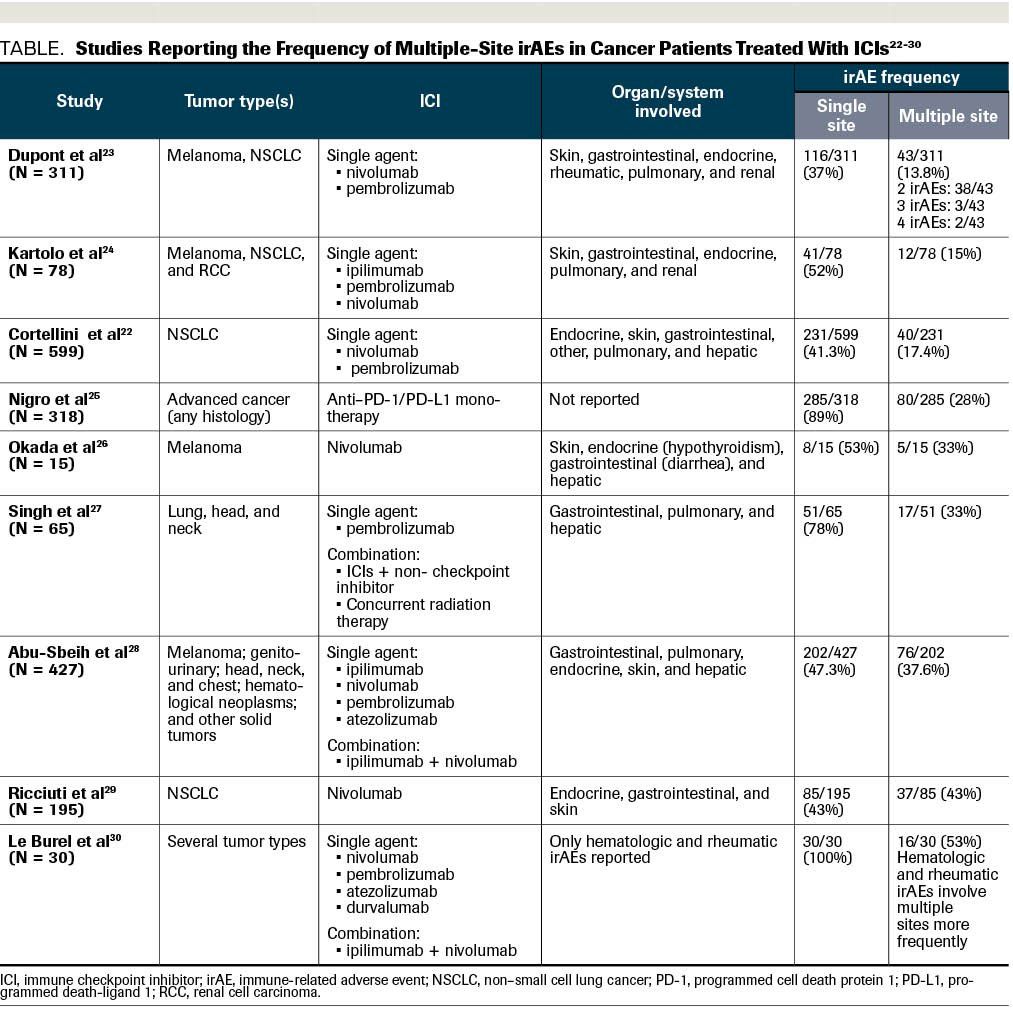
TABLE. Studies Reporting the Frequency of Multiple-Site irAEs in Cancer Patients Treated With ICIs
A 56-year-old white man with a 74 pack-year smoking history presented with macroscopic hematuria and a significant weight loss of 45 pounds in 6 months. His clinical laboratory tests indicated iron deficiency anemia and a computed tomography (CT) scan showed a left kidney tumor, mediastinal lymph nodes, and multiple lung metastases. A percutaneous CT-guided kidney biopsy revealed grade 3 clear cell renal carcinoma based on World Health Organization/International Society of Urologic Pathology classification.
The patient started first-line systemic treatment for intermediate-risk metastatic renal cell carcinoma (mRCC) with combination immunotherapy with nivolumab plus ipilimumab. After 10 days of the first cycle, he presented with a pruritic maculopapular rash covering 20% of his body surface.
The Case
A 56-year-old white man with a 74 pack-year smoking history presented with macroscopic hematuria and a significant weight loss of 45 pounds in 6 months. His clinical laboratory tests indicated iron deficiency anemia and a computed tomography (CT) scan showed a left kidney tumor, mediastinal lymph nodes, and multiple lung metastases. A percutaneous CT-guided kidney biopsy revealed grade 3 clear cell renal carcinoma based on World Health Organization/International Society of Urologic Pathology classification.
The patient started first-line systemic treatment for intermediate-risk metastatic renal cell carcinoma (mRCC) with combination immunotherapy with nivolumab plus ipilimumab.1 After 10 days of the first cycle, he presented with a pruritic maculopapular rash covering 20% of his body surface (Figure 1A). In consensus with the Dermatology Department, we considered this to be a grade 2 cutaneous immune-related adverse event (irAE) based on the Common Terminology Criteria for Adverse Events (CTCAE).2 He received topical steroids and an oral antihistamine for 1 week; however, due to lack of clinical improvement, the patient was reevaluated and clinical laboratory tests were performed. Results showed a creatinine increase of grade 2 per CTCAE (his baseline creatinine was 1.1 mg/dL, which increased 2.3 times) (Figure 2). Nephrology was consulted for work-up of this acute kidney injury. Urinary tract infection and urinary tract obstruction were ruled out and a bland urinary sediment was found. The patient was diagnosed with tubulointerstitial nephritis related to immune checkpoint inhibitors (ICIs).
Our patient had irAEs involving multiple organ sites; therefore, systemic steroids (prednisone 40 mg once daily) were initiated for the grade 2 irAE and ICIs were held. Ten days later, the grade 2 rash resolved (Figure 1B), and renal failure improved to grade 1 with decreased creatinine levels. He started a slow steroid taper over 4 weeks.2
Which of the following are true about irAEs with ICIs?
A. irAEs are more common when an ICI combination is given
B. The incidence of irAEs at multiple sites is at least 10%
C. irAEs usually develop within weeks to 3 months after initiation of ICIs
D. irAEs may be associated with better clinical outcomes
E. All of the Above
CORRECT ANSWER:
E. All of the Above
Discussion
ICIs are associated with a distinctive class of adverse events (AEs), denominated irAEs. The management of these events remains challenging, and most recommendations are based on expert opinion and guidelines for the management of irAEs from several oncology societies.2-5
irAEs could be explained by T-cell function disinhibition; however, the exact mechanisms remain unknown. Some studies suggest that these events are due to a combination of autoreactive T cells, autoantibodies, and cytokine secretion.6-9
The frequency of irAEs varies according to tumor type, disease stage, and agent or agents prescribed. The incidence of irAEs is estimated to be lower with anti-programmed cell death protein 1 (PD-1) or anti-programmed death-ligand 1 (PD-L1) monotherapy (any grade, 58%-85%; grades 3-4, 7%-20%)4,10,11 compared with anti-cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) monotherapy (any grade, 60%-85%; grades 3-4, 10%-27%).12,13 The highest rates of irAEs have been observed with anti–PD-L1 and anti–CTLA-4 combinations (any grade, 93%-95%; grades 3-, 40%-55%).4,14,15 Fatal irAEs are relatively uncommon, with an incidence of 0.3% to 1.3%.11,16,17 Therefore, answer A is true.
Currently, ICIs are the preferred first-line therapy for mRCC,18 either in combination with tyrosine kinase inhibitors19,20 or another ICI.1,21 The phase 3 CheckMate 214 study evaluated nivolumab plus ipilimumab versus sunitinib in patients with untreated mRCC. Among the 550 patients who received nivolumab plus ipilimumab, 46% experienced grade 3 to 4 AEs, 35% required high-dose systemic steroids (≥40 mg of prednisone per day), and 22% of patients developed treatment-related AEs leading to discontinuation. The most common irAEs were increased liver enzymes (2%-3%) and diarrhea (3%).1,21
Although it is generally accepted that patients receiving ICIs and combination ICIs may experience irAEs affecting multiple organ sites, the exact incidence of concurrent or sequential irAEs in the same patient has not been reported in the kidney cancer population. However, in a retrospective study of 559 patients with non–small cell lung cancer (NSCLC) given nivolumab or pembrolizumab, 231 (41%) developed irAEs, and 40 (17.4%) presented multiple-site irAEs. These were defined as immune-related events occurring in different systems/organs in the same patient. The most frequent irAEs of any grade were classified as endocrine, cutaneous, and gastrointestinal (GI). In contrast, the most common grades 3 to 4 irAEs were GI and pulmonary. In the multivariate analysis, no factor was associated with the development of single- or multiple-site irAEs. Overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) were higher among patients with AEs compared with those who did not experience AEs (P <.0001), but no significant differences were observed among those who presented multiple-site events compared with those who had single-site irAEs. Among patients with irAEs, 32 (6.1%) discontinued treatment due AEs, and no statistical differences were found in ORR, PFS, and OS when compared in those who did not discontinue treatment.22
The data regarding the incidence of multiple irAEs in the same patient is very scarce. It comes from heterogenous populations (ie, different tumor types and stages) and retrospective studies. The Table includes data from studies that report the frequency of multiple-site irAEs, which varies from 13% to 53%.22-30 Therefore, answer B is a correct statement.
Similar to incidence, the onset of irAEs highly depends of the type of ICI and whether the ICI is used as monotherapy or in combination. The toxicities associated with ipilimumab appear within the first 2 to 12 weeks of treatment, usually during the induction phase. The onset of AEs with anti–PD-1 drugs is slightly longer, occurring 5 to 15 weeks after the first dose of treatment. Finally, with ICI combinations, toxicities occur earlier and over a more prolonged period of time.4,11,31 Concerning the development of multiple-site irAEs, the mean time since ICI initiation to their development was similar to single-site irAEs (62 vs 78 days, P = .414).28 Answer C is also correct.
Steroids are the mainstay management strategy for irAEs and can be used in conjunction with other immunosuppressive agents according to the evolution and severity of the event. For grade 2 toxicities, ICIs are held, and corticosteroids are initiated at an initial dose of 0.5 to 1 mg/kg per day (prednisone or equivalent). For grade 3 toxicities, ICIs are held, and high-dose corticosteroids are initiated at an initial dose of 1 to 2 mg/kg day (prednisone or equivalent). For grade 4 toxicities, ICIs are permanently discontinued, and high-dose corticosteroids are initiated. Upon response to treatment, corticosteroids are tapered over 4 to 6 weeks. If there is no response, corticosteroids are used in conjunction with other immunosuppressive agents, such as infliximab or mycophenolate, depending on site of irAE and severity.2 irAEs may affect more than 1 organ simultaneously (as in our case) or sequentially. Therefore, immunosuppressive treatment may require a longer duration or to be given at different periods of time. In the case of multiple-site AEs, the MD Anderson Cancer Center experience indicates that treatment type, its duration, and associated complications were comparable between patients with single and multiple irAEs.28
In 2 retrospective studies in patients with mRCC treated with nivolumab after receiving a prior tyrosine kinase inhibitor, OS and PFS were significantly longer in those with irAEs than in those without irAEs. Multivariable analyses revealed a protective association with irAEs in both outcomes in each study.32,33 In addition, an Italian study analyzed patients with NSCLC treated with nivolumab and found that individuals who developed at least 2 irAEs (43%) had longer median PFS and OS compared with those with a single or no irAEs (P <.0001). Among patients with irAEs, 14% (n = 12) discontinued immunotherapy because of AEs, and at the time of analysis, 11 were alive without disease progression or subsequent treatment.29 In lieu of this evidence, there is some suggestion that irAEs may be associated with better clinical outcomes. Therefore, answer D is a correct statement.
Data from the CheckMate 214 study of mRCC treatment in the first-line setting revealed that in the ICI arm, 25% of patients discontinued treatment due to irAEs (median duration of treatment 2.1 months). These patients generally had higher ORRs, complete response rates, and 24-month OS compared with sunitinib. These efficacy outcomes were also similar to the intention-to-treat population in the nivolumab plus ipilimumab arm. The latter suggests that oncological outcomes remain comparable with immunotherapy despite the need for treatment suspension due to toxicity.34
Outcome of This Case
In this case, we decided to continue with a second cycle of the ICI combination while the patient was tapering off steroids (at dose of prednisone 15 mg/day) after 35 days of treatment delay.
Is Re-Treatment With Immunotherapy in this Patient an Option?
Currently, the correct answer remains unclear because the benefit in survival with re-treatment is uncertain and some consider that patients with irAEs experience enhanced ICI efficacy and that re-treatment may be not be necessary. The current guideline recommendations state that for grade 2 and 3 irAEs, when symptoms and/or laboratory values return to grade 1 or less, cautious rechallenge with ICIs may be offered. However, ICIs should be permanently discontinued if patients have grade 4 irAEs, with the exception of endocrinopathies that are controlled with hormone replacement.2 Another option following combination nivolumab and ipilimumab therapy would be a rechallenge with nivolumab only.
In the mRCC setting, there is 1 retrospective cohort analysis where 80 patients with clinically significant irAEs, defined as those requiring treatment interruption or discontinuation, were evaluated. The most common irAEs were transaminitis, colitis, and pneumonitis. In 36 (45%) patients, ICI treatment was restarted. Eleven (30.5%) patients were receiving nivolumab plus ipilimumab (like our patient); 7 (63.5%) patients were switched to nivolumab monotherapy and 4 (36.3%) patients restarted the combination. The initial grade of irAE did not predict whether the patient would be rechallenged with monotherapy or the combination.35 After ICI retreatment, 12 (33.3%) patients developed a new type of irAE and 6 (16.6%) experienced the same one. This implied that 50% of patients with mRCC developed a new or recurrent irAE after restarting treatment, similar to results in patients with NSCLC and melanoma.36,37
Regarding posttreatment AEs, 39% of irAEs were grade 3 with there were no grade 4 or 5 events. Colitis was the most frequent irAE after rechallenge. The median time to an irAE was 2.8 months (range, 0.3-13.8 months), 6 (33.3%) patients required hospitalization, 11 (61.1%) required systemic steroids, and 13 (72%) patients required a therapy interruption for at least 1 week (10 (77%) of which permanently discontinued treatment). The median duration of immunotherapy after retreatment was 5.3 months (range: <1.0 to 21.3 months).35
Two significant predisposing factors for developing irAEs after rechallenge have been identified in patients with metastatic melanoma given combined anti–CTLA-4 and anti–PD-1 therapy. The first is a shorter duration between final dose of combination therapy to resumption of an ICI, and the second is the use of steroids at the time of restart.37
In conclusion, ICIs can cause irAEs involving multiple organ sites in the same patient, concurrently or sequentially. Careful monitoring and dose interruption of ICIs, and early initiation of corticosteroids when needed, are important. Rechallenge with ICIs may be appropriate for some patients but continued monitoring for new or recurrent irAEs is critical.
About the SERIES EDITORS:
Maria T. Bourlon, MD is associate professor, head of the Urologic Oncology Clinic, national researcher. Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. Mexico City, Mexico. She is also a member of ASCO’s IDEA Working Group.
E. David Crawford, MD, is chairman, Prostate Conditions Education Council; editor in chief, Grand Rounds in Urology; and professor of Urology, University of California San Diego, La Jolla, California.
Disclosures:
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Motzer RJ, Tannir NM, McDermott DF, et al; CheckMate 214 Investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290. doi:10.1056/NEJMoa1712126.
2. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714-1768. doi: 10.1200/JCO.2017.77.6385.
3. Puzanov I, Diab A, Abdallah K, et al; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. doi: 10.1186/s40425-017-0300-z.
4. Haanen JBAG, Carbonnel F, Robert C, et al; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv119-iv42. doi: 10.1093/annonc/mdx225.
5. Thompson JA, Schneider BJ, Brahmer J, et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J Natl Compr Canc Netw. 2020;18(3):230â241. doi:10.6004/jnccn.2020.0012
6. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158-168. doi: 10.1056/NEJMra1703481.
7. Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28(3):583-589. doi: 10.1093/annonc/mdw640.
8. Lim SY, Lee JH, Gide TN, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin Cancer Res. 2019;25(5):1557-1563. doi: 10.1158/1078-0432.CCR-18-2795.
9. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755. doi: 10.1056/NEJMoa1609214.
10. Wang Y, Zhou S, Yang F, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008-1019. doi: 10.1001/jamaoncol.2019.0393.
11. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563-580. doi: 10.1038/s41571-019-0218-0.
12. Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8.
13. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723. doi: 10.1056/NEJMoa1003466.
14. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23-34. doi: 10.1056/NEJMoa1504030.
15. Xu H, Tan P, Ai J, et al. Antitumor activity and treatment-related toxicity associated with nivolumab plus ipilimumab in advanced malignancies: a systematic review and meta-analysis. Front Pharmacol. 2019;10:1300. doi: 10.3389/fphar.2019.01300.
16. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86-104. doi: 10.3322/caac.21596.
17. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721-1728. doi: 10.1001/jamaoncol.2018.3923.
18. Hudes G, Carducci M, Tomczak P, et al; Global ARCC Trial. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271-2281. doi: 10.1056/NEJMoa066838.
19. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115. doi: 10.1056/NEJMoa1816047.
20. Rini BI, Plimack ER, Stus V, et al; KEYNOTE-426 Investigators. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127. doi: 10.1056/NEJMoa1816714.
21. Motzer RJ, Rini BI, McDermott DF, et al; CHeckMate 214 invesigators. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20(10):1370-1385. doi: 10.1016/S1470-2045(19)30413-9.
22. Cortellini A, Chiari R, Ricciuti B, et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer. 2019;20(4):237-247 e1. doi: 10.1016/j.cllc.2019.02.006.
23. Dupont R, Bérard E, Puisset F, et al. The prognostic impact of immune-related adverse events during anti-PD1 treatment in melanoma and non-small-cell lung cancer: a real-life retrospective study. Oncoimmunology. 2020;9(1):1682383. doi: 10.1080/2162402X.2019.1682383.
24. Kartolo A, Sattar J, Sahai V, Baetz T, Lakoff JM. Predictors of immunotherapy-induced immune-related adverse events. Curr Oncol. 2018;25(5):e403-e410. doi: 10.3747/co.25.4047.
25. Nigro O, Cortellini A, Giusti R, et al. Incidence and clinical implications of late immune-related adverse events in long responders to PD-1/PD-L1 checkpoint inhibitors: a multicenter study. Ann Oncol. 2019;30(suppl 11):xi20.
26. Okada N, Kawazoe H, Takechi K, et al. Association between immune-related adverse events and clinical efficacy in patients with melanoma treated with nivolumab: a multicenter retrospective study. Clin Ther. 2019;41(1):59-67. doi: 10.1016/j.clinthera.2018.11.004.
27. Singh P, Brito A, Abdulnour R-EE, et al. Defining real-world criteria for immune-related adverse events (irAEs). J Clin Oncol. 2019;37(15_suppl):e14172. doi: 10.1200/JCO.2019.37.15_suppl.e14172 Journal of Clinical Oncology 37, no. 15_suppl.
28. Abu-Sbeih H, Tang T, Ali FS, et al. The impact of immune checkpoint inhibitor-related adverse events and their immunosuppressive treatment on patients’ outcomes. Journal of Immunotherapy and Precision Oncology. 2018;1(1):7-18.
29. Ricciuti B, Genova C, De Giglio A, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479-85. doi: 10.1007/s00432-018-2805-3.
30. Le Burel S, Champiat S, Mateus C, et al. Prevalence of immune-related systemic adverse events in patients treated with anti-programmed cell death 1/anti-programmed cell death-ligand 1 agents: a single-centre pharmacovigilance database analysis. Eur J Cancer. 2017;82:34-44. doi: 10.1016/j.ejca.2017.05.032.
31. Sosa A, Lopez Cadena E, Simon Olive C, Karachaliou N, Rosell R. Clinical assessment of immune-related adverse events. Ther Adv Med Oncol. 2018;10:1758835918764628. doi: 10.1177/1758835918764628.
32. Ishihara H, Takagi T, Kondo T, et al. Association between immune-related adverse events and prognosis in patients with metastatic renal cell carcinoma treated with nivolumab. Urol Oncol. 2019;37(6):355.e21- 355.e29. doi: 10.1016/j.urolonc.2019.03.003.
33. Verzoni E, Carteni G, Cortesi E, et al; Italian Nivolumab Renal Cell Cancer Early Access Program group. Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: the Italian expanded access program. J Immunother Cancer. 2019;7(1):99. doi: 10.1186/s40425-019-0579-z.
34. Tannir NM, Motzer RJ, Plimack ER, et al. Outcomes in patients (pts) with advanced renal cell carcinoma (aRCC) who discontinued (DC) first-line nivolumab + ipilimumab (N+I) or sunitinib (S) due to treatment-related adverse events (TRAEs) in CheckMate 214. J Clin Oncol. 2019;37(7_suppl):581. doi: 10.1200/JCO.2019.37.7_suppl.581.
35. Abou Alaiwi S, Xie W, Nassar AH, et al. Safety and efficacy of restarting immune checkpoint inhibitors after clinically significant immune-related adverse events in metastatic renal cell carcinoma. J Immunother Cancer. 2020;8(1):e000144. doi: 10.1136/jitc-2019-000144.
36. Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res. 2018;6(9):1093-1099. doi: 10.1158/2326-6066.CIR-17-0755.
37. Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2018;29(1):250-255. doi: 10.1093/annonc/mdx642.

2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.