Novel Neoadjuvant Treatment Strategies for Triple-Negative Breast Cancer
Experts review the current landscape and potential use of neoadjuvant chemotherapy with additional novel agents for patients with localized TNBC.
Davis is fellow in hematology and oncology at Northwestern University.

Gradishar is the director of the Maggie Daley Center for Women’s Cancer Care and the chief of Hematology and Oncology and deputy director of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.

TABLE 1 Comparison From Key Reported Phase 2 and 3 Neoadjuvant Immune Checkpoint Inhibitor Trials
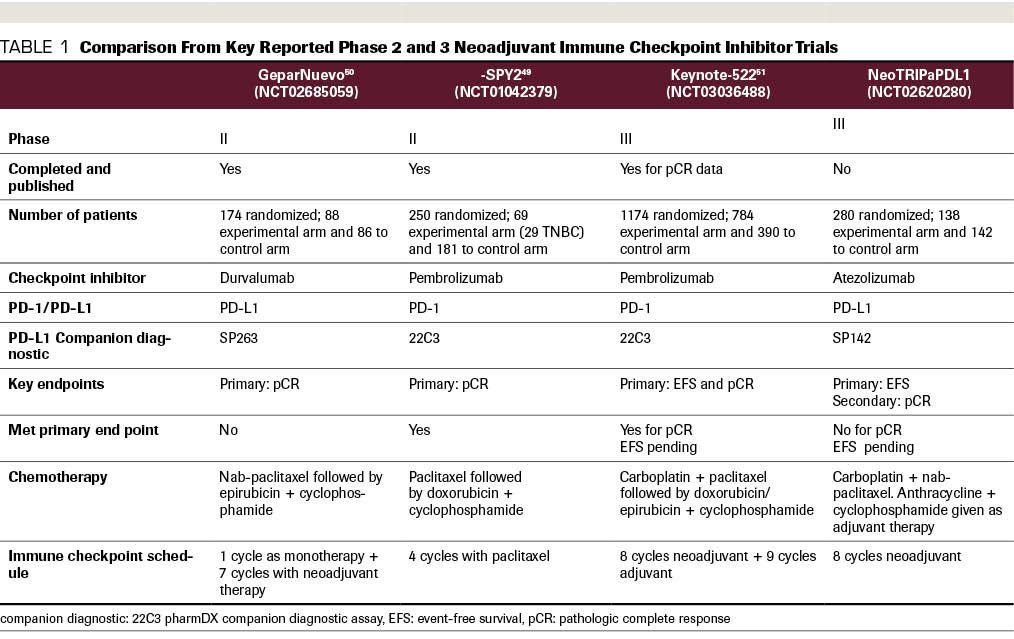
TABLE 2 Select Ongoing Neoadjuvant and Adjuvant Trials of Immune Checkpoint Inhibitors for Localized TNBC
Triple-negative breast cancer (TNBC), which accounts for approximately 10% to 15% of breast cancers, remains the most aggressive subtype and is characterized by early disease relapse for a subset of patients. TNBC remains a clinical challenge, given the lack of effective targeted treatments such as endocrine therapy for hormone receptor–positive (HR+) tumors or therapies against HER2. In contrast to HR+ and HER2+ disease, TNBC remains a diagnosis of exclusion based on the 3 standard immunohistochemistry markers. Despite the aggressive nature of TNBC pathological complete response (pCR) rates of approximately 30% to 40% have been achieved for patients with TNBC with chemotherapy alone, while lower rates are achieved for patients with ER+, luminal A, or luminal B disease. However, despite the higher pCR rates compared with other subtypes, risk of recurrence is considerably higher for patients with TNBC. Despite the significant molecular heterogeneity of the disease, chemotherapy-based regimens have served, until recently, as the primary treatment for these patients, given the lack of reliable molecular targets. Many molecular pathways are being explored in this space as potentially druggable, including DNA repair mechanisms, PI3K/mTOR inhibition, and androgen deprivation. Recently, 2 classes of drugs have become available for a subset of TNBC patients: PARP inhibitors and immune checkpoint inhibitors (ICIs).
Introduction
Triple-negative breast cancer (TNBC), which accounts for approximately 10% to 15% of breast cancers, remains the most aggressive subtype and is characterized by early disease relapse for a subset of patients. TNBC remains a clinical challenge, given the lack of effective targeted treatments such as endocrine therapy for hormone receptor–positive (HR+) tumors or therapies against HER2. In contrast to HR+ and HER2+ disease, TNBC remains a diagnosis of exclusion based on the 3 standard immunohistochemistry markers; it is characterized by the absence of tumor expression for estrogen receptor (ER), progesterone receptor (PR), and HER2. Despite the aggressive nature of TNBC, which is characterized by high-grade tumors with fast cell division and genomic instability, pathological complete response (pCR) rates of approximately 30% to 40% have been achieved for patients with TNBC with chemotherapy alone, while lower rates are achieved for patients with ER+, luminal A, or luminal B disease.1-3 However, despite the higher pCR rates compared with other subtypes, risk of recurrence is considerably higher for patients with TNBC.4 Despite the significant molecular heterogeneity of the disease, chemotherapy-based regimens have served, until recently, as the primary treatment for these patients, given the lack of reliable molecular targets. Many molecular pathways are being explored in this space as potentially druggable, including DNA repair mechanisms, PI3K/mTOR inhibition, and androgen deprivation. Recently, 2 classes of drugs have become available for a subset of TNBC patients: PARP inhibitors and immune checkpoint inhibitors (ICIs).
Prior molecular studies have defined TNBC as a distinct entity with defined subtypes. Specifically, TNBC is classified with basal-like breast cancers (BLBC), which are considered as a distinct molecular subtype from ER+ or HER2+ breast cancers. While not all-encompassing, BLBC represents the most common molecular subtype of TNBC. Other classification systems utilized gene expression profiling to divide TNBC into 6 classifications, including basal-like (BL1 and BL2), mesenchymal (M), mesenchymal stem-like, immunomodulatory, or luminal androgen receptor; this was subsequently revised into just 4 categories: BL1, BL2, M, and LR (for luminal receptor).5 Clinically, the most commonly observed mutations within TNBC are TP53 and PIK3CA, with a diversity of other genomic alterations also encountered at lower frequency.6
The consideration of utilizing a neoadjuvant approach for TNBC relies on several factors. First, upfront therapy enables a better understanding of disease biology and responsiveness to chemotherapy and other systemic agents. Second, this approach is a useful tool in clinical trials to evaluate pathological complete response (pCR) and other surrogate end points at the time of surgery. While achieving pCR is prognostic, there are conflicting data regarding whether pCR can serve as a surrogate end point for improved event-free survival (EFS) or overall survival (OS).1,7,8 Third, similar to other breast cancer subtypes, neoadjuvant treatment may enable pathologic downstaging, decreased need for axillary surgery, and/or improved cosmetic outcome.9 Therefore, this approach is most often utilized in stage II and III disease.
Standard-of-care chemotherapy is recommended for individuals with tumors of at least 1.0 cm (also considered for tumors >0.5 cm) or with lymph node positivity regardless of tumor size. The majority of patients receive anthracycline-, alkylator-, and taxane-based regimens, ideally in a dose-dense approach, despite the heterogeneity of molecular classification.10-12 For patients with smaller, node-negative tumors or patients with medical comorbidity, docetaxel in combination with cyclophosphamide can be considered.13,14 Data for patients with tumors <1.0 cm who do not receive chemotherapy suggest high relapse-free survival rates-at least 90%-with a 5-year survival estimate of 94%.15 Further improvements in survival, while limiting toxicity, are needed for larger and/or node-positive tumors. Here, we review the current landscape and potential use of neoadjuvant chemotherapy with additional novel agents for patients with localized TNBC.
Novel Therapeutic Approaches
Chemotherapy
Given the suboptimal outcomes for patients with localized TNBC, the goal remains to identify novel drug targets to combine with chemotherapy. One strategy that has been studied involves a risk-adapted approach to escalate therapy for patients with residual disease post neoadjuvant therapy. These approaches are feasible with shorter-term follow-up as compared with other breast cancer subtypes, because TNBC recurrences occur earlier than non-TNBC recurrences. An example is the CREATE-X trial (UMIN000000843)that utilized capecitabine for patients with residual disease at the time of surgery. The study authors reported a 13.7% increase in disease-free survival (DFS) with capecitabine with a statistically significant increase in OS rate of 78.8%, compared with 70.3% without capecitabine. In terms of adverse events (AEs), 73.4% of patients developed hand-foot syndrome and more than 40% developed cytopenias. However, these findings were not confirmed as statistically significant in a subsequent phase III trial (GEICAM/CIBOMA).16,17 The latter trial reported no improvement in DFS versus observation. In addition, prior trials utilizing platinums in combination with standard chemotherapy agents have reported modest benefits in pCR rate, with potential greater benefit for patients with BRCA mutations or BRCAness.18-20 However, given conflicting findings in 2 pivotal trials with respect to DFS, phase 3 confirmatory studies are warranted and pending.
PARP Inhibitors
With the emergence of next-generation sequencing, novel targets have been identified for patients with metastatic breast cancer, including the potential to utilize PARP inhibitors, PI3K inhibitors, MEK inhibitors, and HDAC inhibitors, among others. While these pharmaceutical agents have been explored with varied success in metastatic breast cancer, none have been approved as neoadjuvant therapy to date.
PARP inhibitors have been investigated in neoadjuvant trials, particularly for patients with BRCA mutations or BRCAness.21,22 PARP inhibition leads to double-strand breaks that cannot be repaired in patients with homologous recombination deficiency (HRD). PARP inhibitors may serve as chemosensitizers or radiosensitizers and can result in synthetic lethality for patients with germline mutations.
In metastatic disease, the first FDA approvals for PARP inhibitors included olaparib and talazoparib for germline BRCA-mutant, HER2-negative disease based on the OlympiAD and EMBRACA trials, respectively.23,24 The approach of incorporating PARP inhibitors into neoadjuvant trials has also been investigated. For example, the phase 2, single-arm PrECOG 0105 trial reported a high response rate (up to 75%) for patients with BRCA1/2 mutations who received gemcitabine, carboplatin, and iniparib.25 High response rates were also observed for patients with HRD. These findings, however, were not confirmed in a subsequent phase 3 trial, and further data indicated that iniparib may not even inhibit the DNA repair enzyme PARP.26 In the I-SPY 2 trial, adding veliparib and carboplatin to standard chemotherapy, including paclitaxel, doxorubicin, and cyclophosphamide, resulted in a doubling of pCR rate (26% to 51%).27 The phase 3 BrighTNess trial utilized carboplatin and paclitaxel, followed by doxorubicin and cyclophosphamide with or without veliparib.21 For patients with stage II or III TNBC, the study reported that the pCR rate was not significantly higher with the addition of veliparib. In addition, in a cohort of 20 patients with germline BRCA mutations (15 with TNBC), 6 months of neoadjuvant talazoparib for patients with operable TNBC resulted in a residual cancer burden-0 rate of 53%.22
Two important limitations of combining a PARP inhibitor with chemotherapy exist. First, the combination of chemotherapy with an optimal dose of a PARP inhibitor may cause myelosuppression, suggesting the possibility of utilizing PARP inhibitors in a risk-adapted approach in the adjuvant setting. Second, it is unclear whether PARP inhibitors add additional benefit compared with adding a platinum agent alone. Further risk-adapted approaches are being explored to examine the potential benefit of single-agent PARP inhibitors for patients with high-risk features or residual disease at the time of surgery. In addition, preclinical work has suggested synergy for the combination of PIK3 inhibitors with PARP inhibitors.28,29 Such combinations are being studied in early-phase trials in the advanced setting.
There are several ongoing questions regarding the potential clinical utility of PARP inhibitors for TNBC. First, do somatic and germline BRCA mutations confer differential responses to PARP inhibitors? Prior studies for patients with metastatic TNBC receiving PARP inhibitor monotherapy appear to indicate lower response rates for individuals with somatic mutations, indicating the need for combinatorial approaches.30 Second, can DNA repair mutations beyond BRCA1 and BRCA2, such as ATM, RAD51, PALB2, and BARD1, also confer response to PARP inhibitors? For example, ongoing work is examining the potential to utilize talazoparib for BRCA wild-type patients with either a germline or somatic mutation in the HRD pathway.31 Furthermore, a variety of biomarker tests define loss of heterozygosity and allelic imbalance, but more study of clinical utility is needed before applying these tools in routine clinical practice.32,33 Answering these questions will be critical to maximizing the benefit of PARP inhibitors in clinical practice.
Other Molecular Targets
Trials investigating the use of androgen deprivation, PIK3CA inhibitors, MEK inhibitors, and EGFR inhibitors are also being explored. To date, these drugs have been relatively disappointing in terms of clinically meaningful benefit for patients with metastatic TNBC. In terms of androgen blockade, single-arm phase 2 trials have reported a modest clinical benefit rate of 19% with bicalutamide and a small number of complete and partial responses (n = 6; 8% of evaluable subgroup) in a cohort of 78 patients from a total intention-to-treat cohort of 118 patients treated with enzalutamide.34,35 Subsequently, financial support for the phase 3 ENDEAR trial was discontinued. The combination of PIK3CA inhibitors, such as ipatasertib and buparlisib, with chemotherapy have resulted in modest (ipatasertib: 1.3-month increase in PFS; hazard ratio, 0.60; 95% CI, 0.37-0.98) to negligible (buparlisib: 1.2-month decrease in PFS; hazard ratio; 1.18, 95% CI, 0.82-1.68) benefits in PFS for patients with metastatic TNBC (LOTUS and BELLE4 trials, respectively).36,37
In patients with metastatic TNBC, trials have largely been disappointing when exploring activity of other rare targets, including MEK inhibitors and EGFR inhibitors.38-40 Many other novel agents are being studied, including HDAC inhibitors, drugs targeting integrins, and CCR5 antagonists. In addition, the antibody-drug conjugate sacituzumab govitecan-hziy, which targets Trop-2 and delivers a payload that inhibits topoisomerase-1, has recently shown significant activity in metastatic TNBC after multiple lines of therapy.41 Specifically, for TNBC patients who received multiple prior lines of therapy (range, 2-10), the response rate was 33.3%, with a median PFS of 5.5 months and OS of 13.0 months. In April 2020, due to compelling evidence of clinical efficacy, a data safety monitoring committee stopped the phase 3 ASCENT Trial of sacituzumab govitecan-hziy. Formal presentation of the final analyses of the study is pending. As a result of this agent’s promise in the metastatic setting, it will also likely be studied as a neoadjuvant strategy for patients with early-stage TNBC.
Collectively, these data suggest that single-agent, targeted approaches will unlikely be successful treatment strategies. When considering the addition of targeted therapies to standard combination chemotherapy, overlapping toxicities must be considered carefully in order to ensure that adequate chemotherapy can be delivered and prohibitive toxicity is avoided. Similar to the advances in HER2-positive disease, trials designed to adapt therapy based on residual disease at the time of surgery may enable utilization of additional adjuvant therapy for patients with elevated risk of recurrence based on pathologic assessment.
Immune Checkpoint Inhibitors
To date, ICIs have been relatively disappointing in breast cancer as compared with other, more immunogenic tumor types, such as melanoma, renal cell carcinoma, and non–small cell lung cancer. However, of the subtypes of breast cancer, TNBC has demonstrated the most theoretical promise based on high genomic instability, level of tumor-infiltrating lymphocytes (TILs), and PD-L1 expression. As monotherapy for advanced disease, ICIs have resulted in response rates of approximately 5% to 19%, with some indication of higher response rate based on PD-L1 status.42-46 However, median progression-free survival (PFS) has been only about 1.4 to 2.1 months. To date, the first and only FDA approval in breast cancer is for first-line therapy in a subset of patients with advanced TNBC. Specifically, the FDA approved atezolizumab in combination with nab-paclitaxel for individuals with PD-L1 positivity defined by immune cell PD-L1 staining using the Ventana SP142 assay.47
Importantly, there are several differences between primary and metastatic breast cancers. Prior data have demonstrated that primary breast cancers may be more immunogenic. This is based on studies comparing TILs, PD-L1 expression, and immune gene signatures between primary and metastatic disease. This work reported significantly higher TIL counts, PD-L1 expression, and immune activation signatures in primary disease compared with metastatic disease.48
Several studies have recently been published investigating the use of neoadjuvant chemotherapy in combination with immune checkpoint inhibition. Phase 2 data from the I-SPY 2 trial examined pembrolizumab in combination with neoadjuvant chemotherapy (paclitaxel weekly for 12 weeks, followed by 4 cycles of standard doxorubicin and cyclophosphamide every 2-3 weeks) in early-stage breast cancer.49 A total of 4 cycles of pembrolizumab was given during the paclitaxel portion of treatment. The sample included both HR+, HER2-negative (n = 40) and TNBC patients (n = 29) with pCR as the primary end point. Compared with controls receiving standard neoadjuvant chemotherapy, the TNBC patients who received pembrolizumab had a 60% pCR rate compared with 22% for the control arm. Notably, given the design of I-SPY 2, no adaptive changes to the control arm, such as addition of carboplatin to achieve pCR, were counted, which possibly accounted for the lower-than-anticipated pCR rate of only 22% in that arm. In terms of immune-related AEs, diarrhea, thyroid dysfunction, adrenal insufficiency, and pruritus were notably higher in the experimental arm.
In contrast, a randomized, double-blind, placebo-controlled, phase 2 trial of neoadjuvant durvalumab with or without nab-paclitaxel, followed by epirubicin and cyclophosphamide, did not report favorable outcomes with the addition of an ICI.50 This trial included a subgroup of patients treated without a window phase, while the majority of patients (117 of 174) received a single dose of durvalumab or placebo 2 weeks prior to the first dose of nab-paclitaxel as part of a window phase. In the overall population, no significant difference was seen between the experimental and control arms with respect to pCR (53.4% with durvalumab vs 44.2% control; odds ratio (OR), 1.45; 95% CI, 0.80-2.63; P >.20). For patients treated in the window phase, there was a statistically significant improvement in pCR rate (61.0% with durvalumab vs 41.4% control; OR, 2.22; 95% CI, 1.06-4.64; P <.05). However, this subgroup analysis was underpowered. TILs and PD-L1 expression were associated with increased overall response, but they were not predictive of response to durvalumab. The most common immune-related AE was thyroid dysfunction, present in 47% of patients.
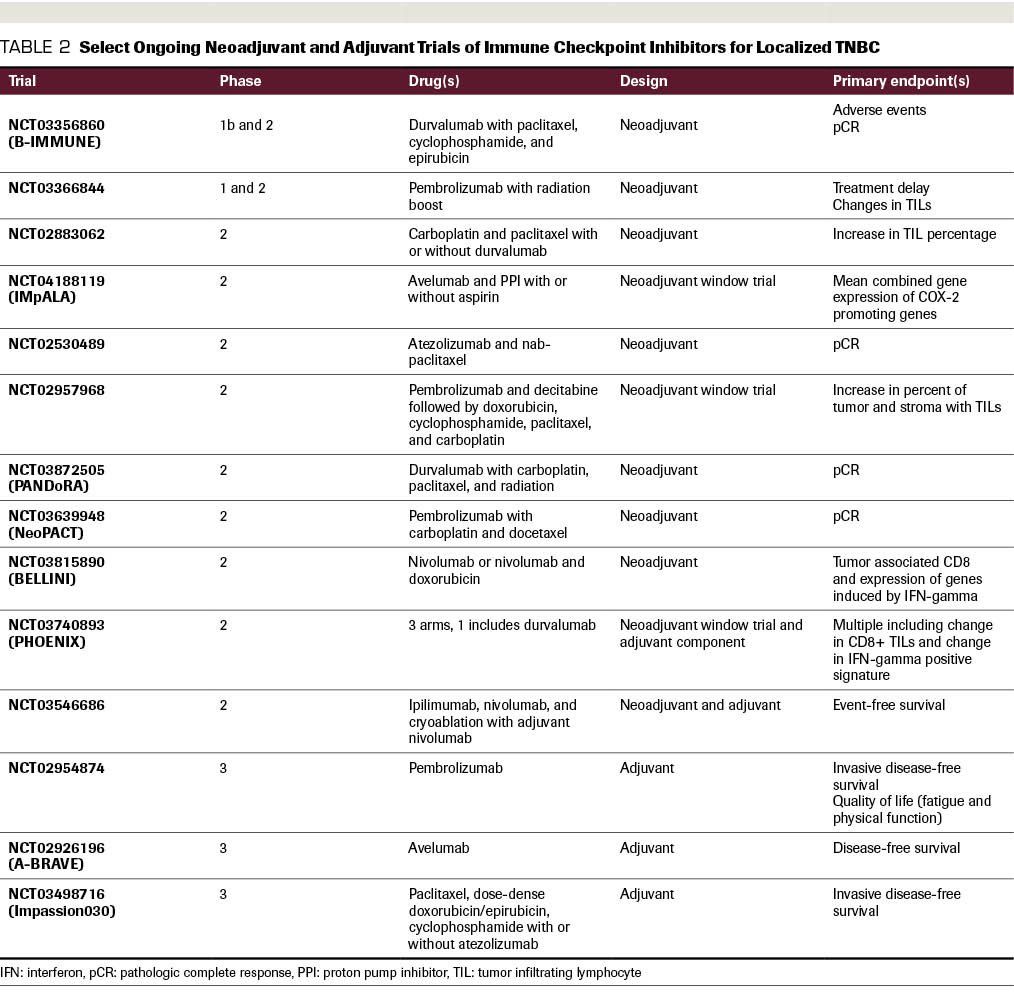
In addition, interim results from 2 phase 3 studies were recently presented at the 2019 San Antonio Breast Cancer Symposium, specifically KEYNOTE-522 (NCT03036488) and NeoTRIPaPDL1 (NCT02620280) (see Table 1 for comparison). KEYNOTE-522 evaluated neoadjuvant pembrolizumab with or without chemotherapy with carboplatin and paclitaxel, followed by an anthracycline (doxorubicin or epirubicin) and cyclophosphamide, followed by 9 cycles of adjuvant pembrolizumab versus placebo. This study included 1174 patients with stage T1c N1-2 or T2-T4 N0-2 TNBC; they were from 21 countries and 124 sites.51 The study reported a significant improvement in pCR rate regardless of PD-L1 status, as revealed by the 22C3 pharmDX companion diagnostic assay for PD-L1 testing. In this study, a combined positive score of 1 or greater was utilized based on the number of PD-L1 positive cells-including tumor cells, lymphocytes, and macrophages-divided by the total tumor cells, then multiplied by 100. Interestingly, tumors with PD-L1 positivity had a higher rate of pCR compared with PD-L1–negative tumors. With short follow-up of 15.5 months, EFS trended in the favorable direction (91.3% with pembrolizumab vs 85.3% in the control arm), but longer-term follow-up is needed to adequately assess statistical differences between the groups for EFS.
In contrast, the NeoTRIPaPDL1 study (NCT02620280) did not report a favorable outcome with a neoadjuvant ICI with respect to pCR.52 This study enrolled high-risk patients with early-stage or locally advanced disease to receive carboplatin and nab-paclitaxel with or without atezolizumab for 8 cycles. In this trial, the anthracycline and cyclophosphamide were given as postoperative therapy. Based on initial evaluation of 280 patients, there was no statistically significant difference in pCR rate (43.5% vs 40.8%; OR, 1.11; 95% CI, 0.69-1.79). PD-L1–positive tumors (using the SP142 companion diagnostic) had a higher rate of pCR compared with PD-L1–negative tumors, but PD-L1 positivity did not predict which patients benefited. PD-L1 positivity was the only variable in multivariate analysis to significantly increase pCR rate. Serious AEs were significantly higher in the experimental arm (18.1%) versus without atezolizumab (5.7%).
Several hypotheses have been posited to account for differences in the results of these 2 phase 3 trials. First, the KEYNOTE-522 trial utilized pembrolizumab both as neoadjuvant and adjuvant therapy, and this may have enhanced the effect of the checkpoint inhibition. The trend toward improved EFS could at least partially be explained by the adjuvant use of pembrolizumab. Second, an anthracycline was given prior to surgery in KEYNOTE-522 and after surgery in NeoTRIPaPDL1. Prior data have indicated that doxorubicin was the most immunogenic chemotherapy backbone to prime the tumor microenvironment.53 Third, the NeoTRIPaPDL1 study enrolled patients with more advanced tumors, and therefore the patients with defined PD-L1 positivity were lower by nearly 30%, taking into account differences in the companion diagnostics utilized in each study. Fourth, various studies define PD-L1 positivity differently based on thresholds, cell type examined, and companion diagnostics.54 All of these factors may explain the discordant results that were observed.
In the future, more clearly defined biomarkers are required to help distinguish which patients may be more likely to respond to therapy. Unfortunately, PD-L1 status does not appear to be a predictive biomarker of pCR in this setting. In addition, further work is necessary to characterize the optimal chemotherapy backbone to combine with ICIs. The particular drugs and sequence of therapy may be very important for optimal patient outcomes. Toxicity and cost data will also be important considerations, given that some immune-related AEs are permanent and a small subset of these toxicities can be severe and even life-threatening. Finally, and most important, longer-term follow-up data regarding EFS, OS, and quality of life are truly necessary to verify that improvements in pCR will correlate with long-term outcomes that matter to patients.
Multiple additional studies utilizing ICIs are ongoing; some representative studies are shown in Table 2. The proposed strategies include a variety of approaches.
For example, some trials are studying the use of ICIs as sequential versus concurrent therapy, while others are exploring adjuvant ICIs for patients with residual disease at the time of surgery. Multiple trials are performing correlative studies examining TILs, interferon-γ immune signatures, and other biomarkers to better understand responders versus nonresponders.
Conclusions
Improving outcomes for patients with localized TNBC remains a significant unmet need in the breast cancer community. Given the fast rate of cell division, chemotherapy will likely remain a backbone of treatment. Therefore, the goal remains to add additional targeted and/or immune-based treatment, while balancing efficacy and minimizing compounding toxicities for patients with the goal of cure. To date, early evidence from some studies has indicated that the combination of standard chemotherapy with immune checkpoint inhibition may improve pCR rates. Longer-term data with respect to EFS and OS are necessary to confirm that pCR will be validated as a surrogate end point in this setting. The optimal timeframe of neoadjuvant and/or adjuvant initiation of ICIs requires further study. At this time, the FDA has not approved the use of ICIs as neoadjuvant therapy, and these therapies should be viewed as experimental, pending final review and analysis of longer-term data. Evaluation of targeted therapies in combination with chemotherapy and/or immune checkpoint inhibition is also being explored in this setting. Collectively, recent data indicate that novel neoadjuvant approaches with ICIs hold promise for patients with TNBC, while we await long-term follow-up data and take into careful consideration the cost and added short- and long-term toxicities of these approaches.
Disclosures:
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References:
1. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. doi:10.1016/S0140-6736(13)62422-8
2. von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796-1804. doi:10.1200/JCO.2011.38.8595
3. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938-1948. doi:10.1056/NEJMra1001389
4. Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275-1281. doi:10.1200/JCO.2007.14.4147
5. Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750-2767. doi:10.1172/JCI45014
6. Bertucci F, Ng CKY, Patsouris A, et al. Genomic characterization of metastatic breast cancers. Nature. 2019;569(7757):560-564. doi:10.1038/s41586-019-1056-z
7. Symmans WF, Wei C, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35(10):1049-1060. doi:10.1200/JCO.2015.63.1010
8. Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366(26):2438-2441. doi:10.1056/NEJMp1205737
9. Hayes DF, Schott AF. Neoadjuvant chemotherapy: what are the benefits for the patient and for the investigator? J Natl Cancer Inst Monogr. 2015;2015(51):36-39. doi:10.1093/jncimonographs/lgv004
10. Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21(8):1431-1439. doi:10.1200/JCO.2003.09.081. Published correction appears in J Clin Oncol. 2003;21(11):2226.
11. Gluz O, Nitz UA, Harbeck N, et al; West German Study Group. Triple-negative high-risk breast cancer derives particular benefit from dose intensification of adjuvant chemotherapy: results of WSG AM-01 trial. Ann Oncol. 2008;19(5):861-870. doi:10.1093/annonc/mdm551
12. Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27(8):1168-1176. doi:10.1200/JCO.2008.18.1024
13. Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24(34):5381-5387. doi:10.1200/JCO.2006.06.5391
14. Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in early breast cancer: the ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol. 2017;35(23):2647-2655. doi:10.1200/JCO.2016.71.4147
15. Vaz-Luis I, Ottesen RA, Hughes ME, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol. 2014;32(20):2142-2150. doi:10.1200/JCO.2013.53.1608
16. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi:10.1056/NEJMoa1612645
17. Lluch A, Barrios CH, Torrecillas L, et al; GEICAM Spanish Breast Cancer Grouop; CIBOMA (Iberoamerican Coalition for Research in Breast Oncology); and LACOG (Latin American Cooperative Oncology Group). Phase III trial of adjuvant capecitabine after standard neo-/adjuvant chemotherapy in patients with early triple-negative breast cancer (GEICAM/2003-11_CIBOMA/2004-01). J Clin Oncol. 2020;38(3):203-213. doi:10.1200/JCO.19.00904. Published correction appears in J Clin Oncol. 2020;38(8):847. doi:10.1200/JCO.20.00164
18. Denkert C, Liedtke C, Tutt A, von Minckwitz G. Molecular alterations in triple-negative breast cancer--the road to new treatment strategies. Lancet. 2017;389(10087):2430-2442. doi:10.1016/S0140-6736(16)32454-0
19. Byrski T, Dent R, Blecharz P, et al. Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer. Breast Cancer Res. 2012;14(4):R110. doi:10.1186/bcr3231
20. Godet I, Gilkes DM. BRCA1 and BRCA2 mutations and treatment strategies for breast cancer. Integr Cancer Sci Ther. 2017;4(1). doi:10.15761/ICST.1000228
21. O’Leary B, Cutts RJ, Liu Y, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 2018;8(11):1390-1403. doi:10.1158/2159-8290.CD-18-0264
22. Litton JK, Scoggins ME, Hess KR, et al. Neoadjuvant talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant. J Clin Oncol. 2020;38(5):388-394. doi:10.1200/JCO.19.01304
23. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523-533. doi:10.1056/NEJMoa1706450. Published correction appears in N Engl J Med. 2017;377(17):1700. doi:10.1056/NEJMx170012
24. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753-763. doi:10.1056/NEJMoa1802905
25. Telli ML, Jensen KC, Vinayak S, et al. Phase II study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer with assessment of a tumor-based measure of genomic instability: PrECOG 0105. J Clin Oncol. 2015;33(17):1895-1901. doi:10.1200/JCO.2014.57.0085
26. Sinha G. Downfall of iniparib: a PARP inhibitor that doesn’t inhibit PARP after all. J Natl Cancer Inst. 2014;106(1):djt447. doi:10.1093/jnci/djt447
27. Rugo HS, Olopade OI, DeMichele A, et al; I-SPY 2 Investigators. Adaptive randomization of veliparib-carboplatin treatment in breast cancer. N Engl J Med. 2016;375(1):23-34. doi:10.1056/NEJMoa1513749
28. Juvekar A, Burga LN, Hu H, et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012;2(11):1048-1063. doi:10.1158/2159-8290.CD-11-0336
29. Ibrahim YH, GarcÃa-GarcÃa C, Serra V, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012;2(11):1036-1047. doi:10.1158/2159-8290.CD-11-0348
30. Pilié PG, Gay CM, Byers LA, O’Connor MJ, Yap TA. PARP inhibitors: extending benefit beyond BRCA-mutant cancers. Clin Cancer Res. 2019;25(13):3759-3771. doi:10.1158/1078-0432.CCR-18-0968.
31. Gruber JJ, Afghahi A, Hatton A, et al. Talazoparib beyond BRCA: a phase II trial of talazoparib monotherapy in BRCA1 and BRCA2 wild-type patients with advanced HER2-negative breast cancer or other solid tumors with a mutation in homologous recombination (HR) pathway genes. J Clin Oncol. 2019;37(15_suppl):3006-3006. doi:10.1200/JCO.2019.37.15_suppl.3006
32. Abkevich V, Timms KM, Hennessy BT, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107(10):1776-1782. doi:10.1038/bjc.2012.451
33. Popova T, Manié E, Rieunier G, et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 2012;72(21):5454-5462. doi:10.1158/0008-5472.CAN-12-1470
34. Gucalp A, Tolaney S, Isakoff SJ, et al; Translational Brerast Cancer Research Consortium (TBCRC 011). Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013;19(19):5505-5512. doi:10.1158/1078-0432.CCR-12-3327
35. Traina TA, Miller K, Yardley DA, et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. 2018;36(9):884-890. doi:10.1200/JCO.2016.71.3495
36. Kim SB, Dent R, Im SA, et al; LOTUS Investigators. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372. doi:10.1016/S1470-2045(17)30450-3
37. Martin M, Chan A, Dirix L, et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4). Ann Oncol. 2017;28(2):313-320. doi:10.1093/annonc/mdw562
38. Infante JR, Papadopoulos KP, Bendell JC, et al. A phase 1b study of trametinib, an oral mitogen-activated protein kinase kinase (MEK) inhibitor, in combination with gemcitabine in advanced solid tumours. Eur J Cancer. 2013;49(9):2077-2085. doi:10.1016/j.ejca.2013.03.020
39. Baselga J, Gómez P, Greil R, et al. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2013;31(20):2586-2592. doi:10.1200/JCO.2012.46.2408. Published correction appears in J Clin Oncol. 2018;36(1):98. doi:10.1200/JCO.2017.76.9976
40. Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012;30(21):2615-2623. doi:10.1200/JCO.2010.34.5579
41. Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741-751. doi:10.1056/NEJMoa1814213
42. Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460-2467. doi:10.1200/JCO.2015.64.8931
43. Rugo HS, Delord JP, Im SA, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res. 2018;24(12):2804-2811. doi:10.1158/1078-0432.CCR-17-3452
44. Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397-404. doi:10.1093/annonc/mdy517
45. Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167(3):671-686. doi:10.1007/s10549-017-4537-5
46. Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5(1):74-82. doi:10.1001/jamaoncol.2018.4224
47. Schmid P, Adams S, Rugo HS, et al; IMpassion130 Trial Investigators. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108-2121. doi:10.1056/NEJMoa1809615
48. Szekely B, Bossuyt V, Li X, et al. Immunological differences between primary and metastatic breast cancer. Ann Oncol. 2018;29(11):2232-2239. doi:10.1093/annonc/mdy399
49. Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. Published online February 13, 2020. doi:10.1001/jamaoncol.2019.6650
50. Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30(8):1279-1288. doi:10l1093/annonc/mdz158
51. Schmid P, Cortes J, Pusztai L, et al; KEYNOTE-522 Investigators. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810-821. doi:10.1056/NEJMoa1910549
52. Gianni L, Huang C-S, Egle D, et al. GS3-04: pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomized study. Abstract presented at: 2019 San Antonio Breast Cancer Symposium; December 10-14, 2019; San Antonio, TX. Accessed April 30, 2020. https://www.abstractsonline.com/pp8/#!/7946/presentation/1911.
53. Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25(6):920-928. doi:10.1038/s41591-019-0432-4. Published correction appears in Nat Med. 2019;25(7):1175. doi:10.1038/s41591-019-0520-5
54. Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):278. doi:10.1186/s40425-019-0768-9
