Isolated Extramedullary Relapse in Acute Lymphoblastic Leukemia: What Can We Do Before and After Transplant?
The case of a 43-year-old male with a history of B-cell acute lymphoblastic leukemia.
Dr. Acosta-Medina is a Research Intern at the Department of Hematology and Oncology at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán from the Universidad Panamericana School of Medicine.

Dr. García-Miranda is an Internal Medicine physician and Hematology Fellow at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubiran.

Dr. Inclan-Alarcon is a Hematologist at the Division of Hematology and Bone Marrow Transplantation in the ABC Medical Center in Mexico City. He is a clinician, invited professor of the Internal Medicine program at his institution and professor of hematology in the School of Medicine at Universidad Anahuac.

Dr. Riviello-Goya is the Chief Resident of Internal Medicine at the Instituto Nacional de Ciencias Médicas y Nutrición and aspiring Hematology Fellow

Dr. Bourlon is an Assistant Professor of Hematology and Bone Marrow Transplant in the Department of Hematology and Oncology at the Salvador Zubirán National Institute of Medical Sciences and Nutrition, Mexico City, Mexico.

Figure 1 Light microscopy of Wright-stained CSF showing uncountable lymphoid-appearing blasts.
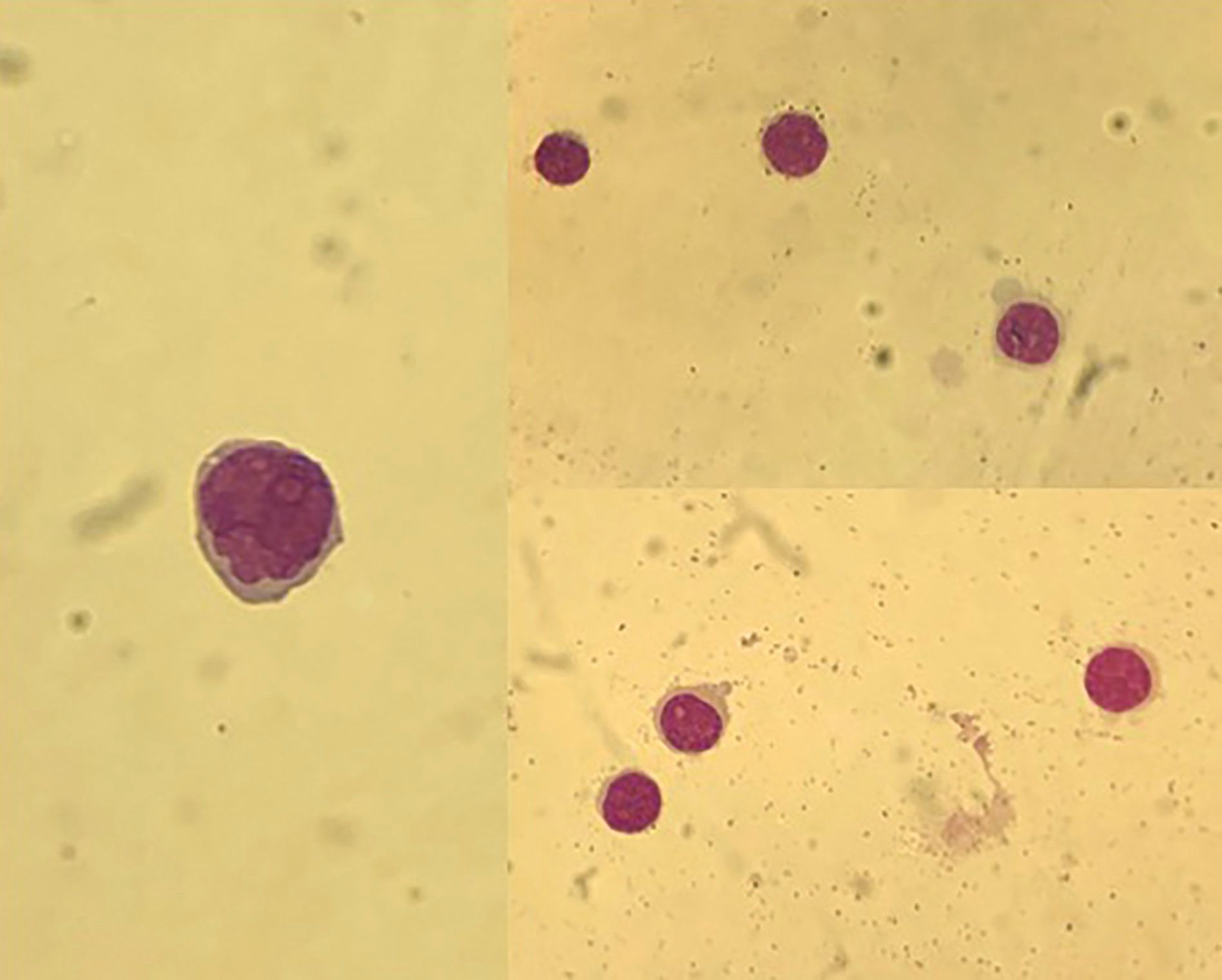
Figure 2 Flow cytometry of CSF showing a CD34+, CD45+, CD19+, CD22+, and CD10+ population (blue) consistent with CNS infiltration by B-lineage acute leukemia.
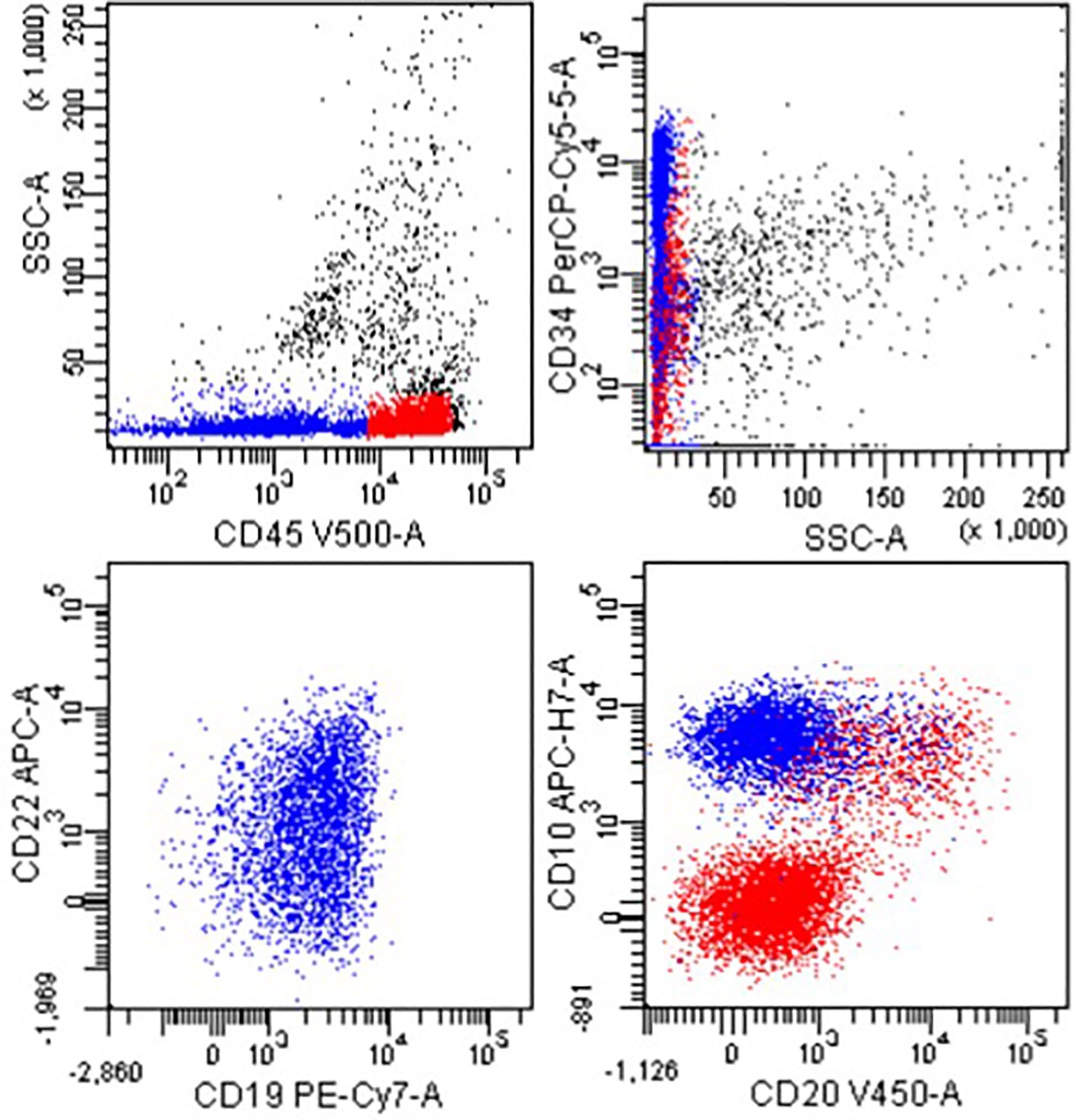
Figure 3 Ultrasound of the testes showing diffuse bilateral hypoechogenicity and increased vascularity, suggestive of leukemic infiltration.
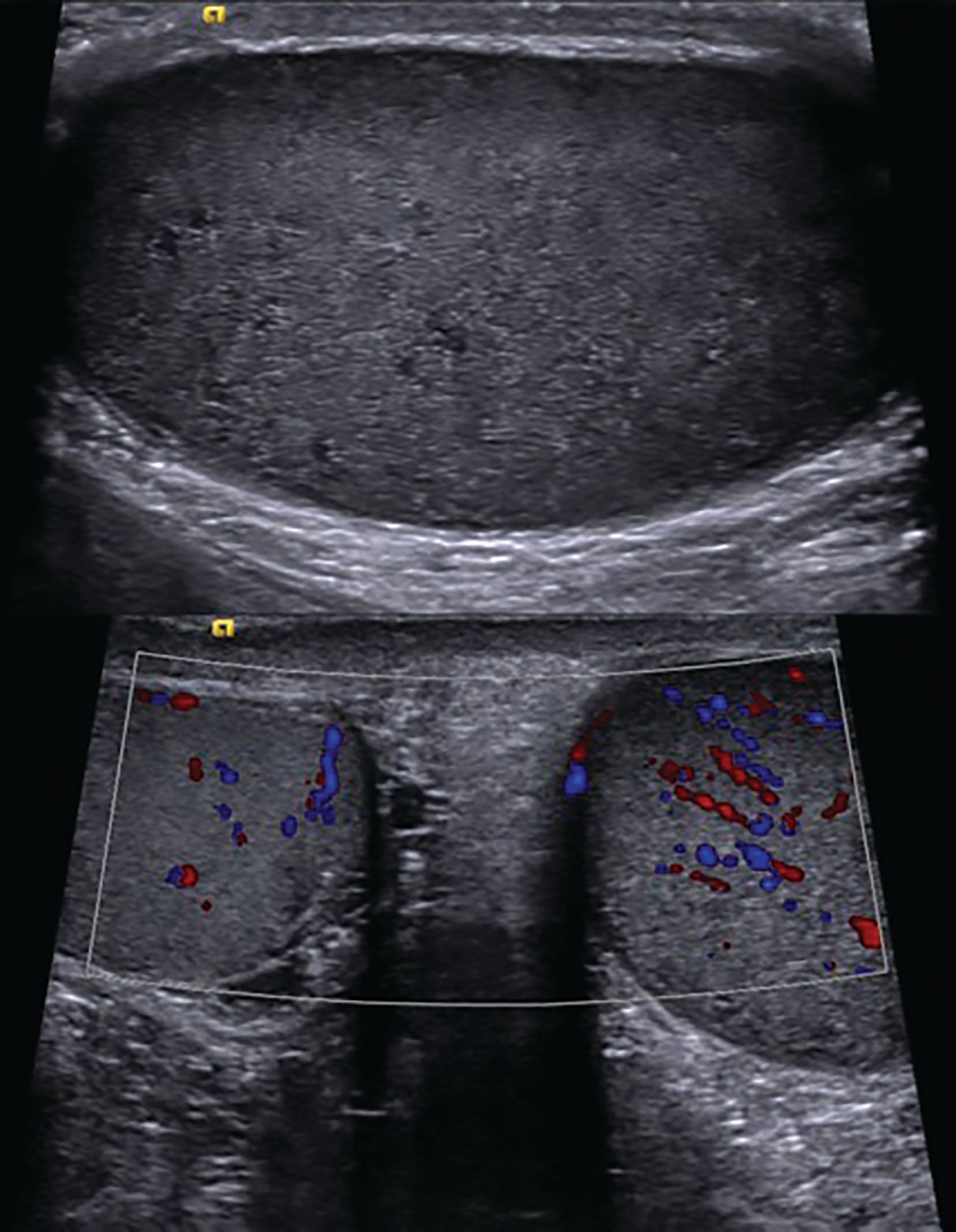
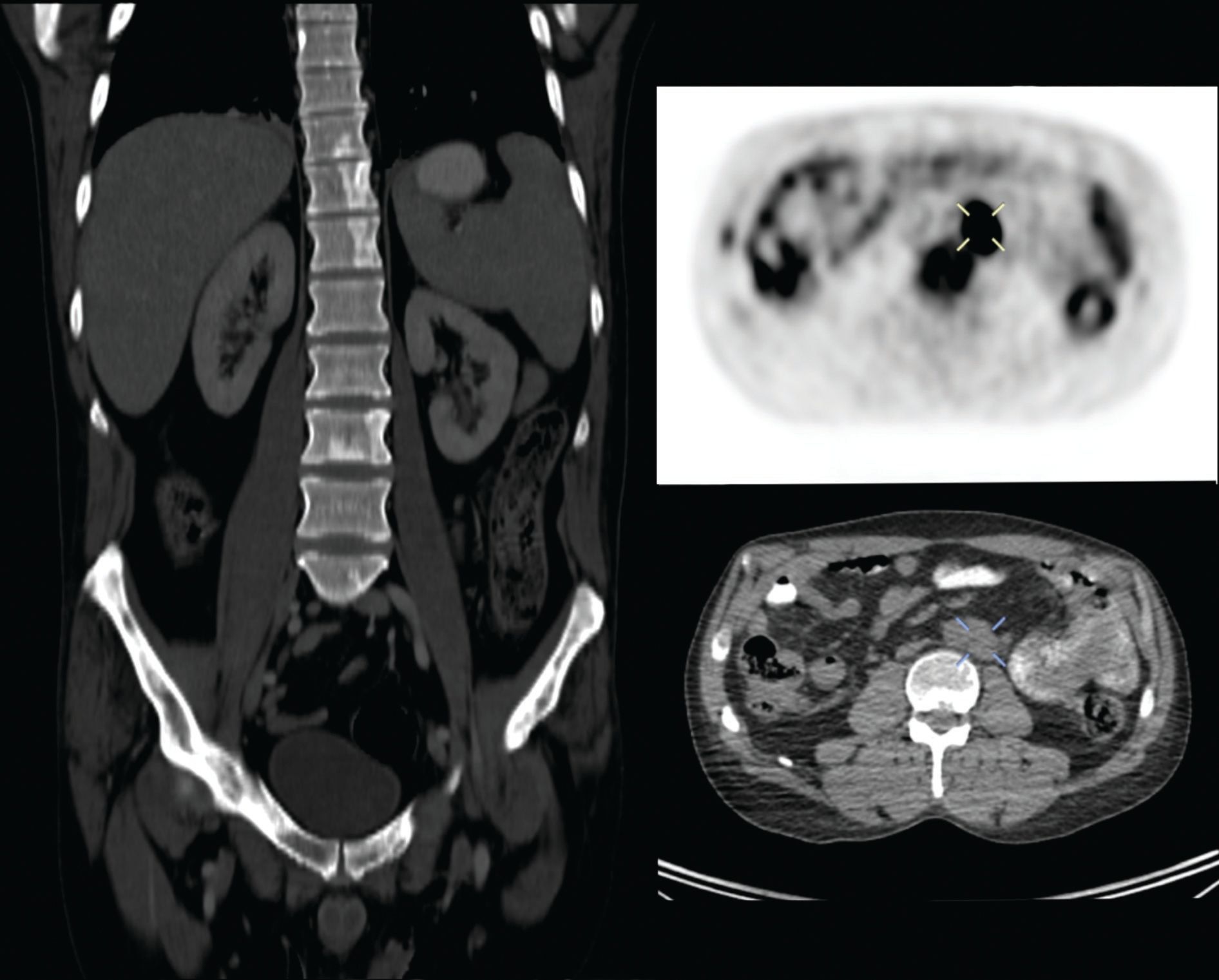
Figure 4 PET-CT imaging one-year post-relapse evidencing osseous lesions and lymphadenopathy.
KEY POINTS
• Isolated EMR is defined as the presence of clonal blasts in any tissue other than the medullary compartment with a bone marrow evaluation with <5% clonal blasts and a full donor chimerism.
• Patients with iEMR have shown better survival outcomes when compared to BMR and EMR and in most cases it heralds a systemic relapse.
• Risk factors for iEMR include: younger age, history of EMD, poor risk cytogenetics, advanced disease at HSCT, development of GVHD, and non-TBI based conditioning regimens.
• Combination therapy, local and systemic, can achieve better remission rates in this subgroup of patients.
Santiago Riviello-Goya, MD1; Aldo A. Acosta-Medina, MD2; Sergio I. Inclan-Alarcon, MD3; SofÃa Garcia-Miranda, MD2; and Christianne Bourlon, MD, MHSc2
1Department of Medicine, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico; 2Department of Hematology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico; 3Cancer Center, Centro Médico ABC, Mexico City, Mexico
THE CASE
A 43-year-old male with a history of B-cell acute lymphoblastic leukemia (ALL), who underwent allogeneic hematopoietic stem cell transplantation (HSCT) 5 months prior, presented to the emergency department with a 5-day history of progressive bilateral lower extremity weakness. On physical examination, there were no additional neurologic findings; sensory function and urethral and anal sphincter tone were preserved.
Initial clinical laboratory testing showed peripheral blood cell counts, a peripheral blood smear, and a comprehensive metabolic panel within normal limits. Neuroimaging by computed tomography (CT) and magnetic resonance showed no evidence of acute intracranial processes or lesions suggestive of leukemic relapse. A lumbar puncture for cerebrospinal fluid (CSF) analysis was performed and documented the presence of lymphoid-appearing blasts (Figure 1). Flow cytometry (FC) confirmed central nervous system (CNS) infiltration by B-lineage lymphoid blasts (CD34+, CD45+, CD22+, CD19+, and CD10+) (Figure 2). Bone marrow aspirate and biopsy, including FC evaluation, were negative for systemic relapse. Bone marrow chimerism was 98%.
With a diagnosis of isolated extramedullary leukemic relapse (iEMR), the patient was initiated on weekly intrathecal chemotherapy and was weaned off graft-versus-host disease (GVHD) prophylaxis, achieving CSF clearance after 4 weeks of therapy. Against Hematology service recommendations, the patient declined systemic therapy and received only whole brain radiation therapy (24 Gy in 12 fractions).
The patient experienced remission of neurologic symptoms; however, after 5 months, he developed bilateral testicular tenderness and enlargement. An ultrasound was performed and was suggestive of leukemic infiltration (Figure 3). Chemotherapy with methotrexate and L-asparaginase in addition to radiotherapy to the testes (24 Gy in 12 fractions) was given without complications.
One year after initial CNS iEMR, the patient developed overt bone marrow relapse (BMR), as evidenced by development of bone pain throughout the lumbosacral region, and the appearance of multiple blastic and lytic lesions throughout the appendicular and axial skeleton. A positron emission tomography-CT scan documented abdominal lymphadenopathy (Figure 4). With this rapidly progressive picture, the patient was transitioned to supportive care and died 2 months later.
Is the risk of iEMR following HSCT modified by the choice of conditioning regimen? If so, which of the following approaches would have been the best choice to prevent iEMR in this patient?
A. There is no role of conditioning therapy in preventing iEMR
B. Reduced intensity of regimen to favor graft-versus-leukemia (GVL) effect
C. Nonmieloablative regimens including fludarabine
D. Mieloablative regimens including total body irradiation (TBI)
CORRECT ANSWER: D. Mieloablative regimens including total body irradiation (TBI).
Discussion
Allogeneic HSCT is an effective treatment for ALL, which can achieve long-term remission and even a potential cure.1 Antineoplastic activity is dependent on both high-dose chemotherapy and graft alloreactivity, with the latter manifested in the GVL effect, and undesirably yet inherently, in GVHD.2 Despite recent advances in allogeneic HSCT strategies, disease relapse is common and remains the most important cause of death in this population. Relapse is reported in 30% to 40% of patients but can increase to 60% in patients who are in a second complete remission (CR) at time of HSCT.2,3
Risk factors for relapse in patients with ALL who have undergone HSCT include disease- and transplant-related features. Reported high-risk disease characteristics include: hyperleukocytosis at diagnosis (white blood cell count >30 x109/L for B-lineage ALL and >100 x109/L for T-lineage ALL); cytogenetics associated with poor outcomes, including chromosome 11 translocations and t(9;22); a short remission timespan; more than a first CR; and a failed or delayed remission after induction therapy.4 In the HSCT population, transplant-related factors should be considered, including alternative donors other than those who are matched related and matched unrelated, the type of conditioning regimen, and the development of GVHD.2
ALL relapse following HSCT most commonly involves the medullary compartment, with a cumulative incidence of 41% at 5 years. Conversely, extramedullary relapse (EMR) is uncommon, with a 5-year cumulative incidence of 11.0% and 5.8% for EMR and iEMR, respectively.5 Due to the rarity of EMR, its prognostic impact remains controversial and the ideal management strategies are a subject of active study.
EMR is associated with poor clinical outcomes; however, the subgroup of patients with iEMR (as presented in this patient case) is gaining attention due to its increasing frequency, its role heralding a systemic relapse, and its clinical behavior showing better survival outcomes compared with BMR and EMR.6-8
Isolated EMR is defined as the presence of clonal blasts in any tissue other than the medullary compartment; bone marrow evaluation must show less than 5% of clonal blasts and a full donor chimerism. Most commonly affected sites include the skin, soft tissues, lymph nodes, and immune sanctuaries including the CNS and testes.1,5,9 Because prevention rather than treatment of relapse is related to improved survival outcomes, it is important to define subgroups of patients who may benefit fromearly intervention with a personalized transplant strategy.
Higher rates of iEMR have been linked to patients of younger age. This is thought to be secondary to: (1) a higher incidence of ALL compared with acute myeloid leukemia (AML) in this age subgroup, the former of which is most associated with EMR; (2) the relative overrepresentation of myelomonocytic/monocytic phenotypes in AML presenting in young individuals; and (3) the higher likelihood of a history of EMR in children compared with adults.1,10
A history of extramedullary (EM) disease, which has consistently been found to impact the development of iEMR, is preexistent in up to half of patients. In 2 out of 3 cases of EMR, disease affects the site of original EM involvement, possibly due to low efficacy of both high-dose chemotherapy and the GVL effect.1,5 An exception to this is CNS involvement, despite being a risk factor for subsequent CNS iEMR, which is commonly reported de novo, reflecting the protective effect of regularly administered prophylaxis to patients at high risk of CNS infiltration.11
The effect of GVHD on risk of iEMR is highly nuanced. Despite its well-known role as a protective factor for BMR, the same effect does not appear to hold true for iEMR.12 Initial reports in this population showed no differences in relapse-free survival regardless of acute or chronic GVHD (cGVHD) or a positive association between extensive cGVHD and iEMR development.10,13 This has led to investigators to postulate that the underlying physiopathology differs among different types of relapse, with decreased expression of human leukocyte antigen (HLA) minor histocompatibility antigens and adhesion molecules and decreased penetration of both immune cells and high-dose chemotherapy to EM sites.14 These mechanisms lead to decreased effectivness of T-cell dependent cytotoxicity of donor lymphocytes as compared with the medullary compartment, with subsequent clone selection and escape, enabling the development of iEMR.6
With the increased use of alternative donors, this has been contested in the haploidentical setting, with a recent report showing significantly increased rates of iEMR in patients who do not develop cGVHD. It is suggested that the role of GVL, coupled with GVHD, in this HLA-mismatched setting could partially explain the added benefit of GVHD in this subgroup. This report also evidenced increased tumor chemosensitivity in patients with EMR compared with BMR, possibly explained by reduced concentrations of conditioning therapy at EM sites.9
Cytogenetics associated with poor outcomes and advanced disease at the time of HSCT were described as risk factors for iEMR in initial cohort studies.1,5,10,15,16 However, recent publications that include alternative-donor HSCT recipients have reported that a haploidentical source could overcome this negative impact.9
The influence of type of conditioning regimen on likelihood of iEMR has been studied only retrospectively, mainly comparing TBI-based versus chemotherapy-based approaches. The landmark paper by Simpson et al showed a significantly elevated rate of iEMR in patients receiving busulfan-based conditioning. This finding has been related to the lack of penetration of drugs into the immune sanctuaries with chemotherapy-only regimens.17
Multiple approaches, including combination and single treatment for iEMR, have been described. Combination therapy including systemic chemotherapy plus local radiotherapy (or in CNS disease, radiation to the craniospinal axis, intrathecal chemotherapy, and systemic chemotherapy) has been associated with higher response rates than single-treatment strategies.9 Nonetheless, the best responses have been observed when combination therapy is followed by a cellular therapy (eg, second allogeneic HSCT, donor leukocyte infusion, and donor stem cell infusion), leading to CR rates of greater than 80%.5,13 Whether this increase in CR rate translates to an increase in survival outcomes remains debatable due to conflicting results in the current literature for iEMR.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
Corresponding author:
Christianne Bourlon, MD, MHScâ¨Vasco de Quiroga No. 15.â¨Belisario DomÃnguez Sección XVI
Tlalpan, C.P. 14080, Ciudad de México, México
E-mail: chrisbourlon@hotmail.com
References:
1. Ge L, Ye F, Mao X, et al. Extramedullary relapse of acute leukemia after allogeneic hematopoietic stem cell transplantation: different characteristics between acute myelogenous leukemia and acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2014;20(7):1040-1047. doi: 10.1016/j.bbmt.2014.03.030.
2. Pavletic SZ, Kumar S, Mohty M, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on the Epidemiology and Natural History of Relapse following Allogeneic Cell Transplantation. Biol Blood Marrow Transplant. 2010;16(7):871-890. doi: 10.1016/j.bbmt.2010.04.004.
3. Devillier R, Crocchiolo R, Etienne A, et al. Outcome of relapse after allogeneic stem cell transplant in patients with acute myeloid leukemia. Leuk Lymphoma. 2013;54(6):1228-1234. doi: 10.3109/10428194.2012.741230.
4. Hoelzer D, Bassan R, Dombret H, Fielding A, Ribera JM, Buske C; ESMO Guidelines Committee. Acute lymphoblastic leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v69-v82. doi: 10.1093/annonc/mdw025.
5. Shem-Tov N, Saraceni F, Danylesko I, et al. Isolated extramedullary relapse of acute leukemia after allogeneic stem cell transplantation: different kinetics and better prognosis than systemic relapse. Biol Blood Marrow Transplant. 2017;23(7):1087-1094. doi: 10.1016/j.bbmt.2017.03.023.
6. Lee JH, Choi SJ, Lee JH, et al. Anti-leukemic effect of graft-versus-host disease on bone marrow and extramedullary relapses in acute leukemia. Haematologica. 2005;90(10):1380-1388.
7. Xie N, Zhou J, Zhang Y, Yu F, Song Y. Extramedullary relapse of leukemia after allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore). 2019;98(19):e15584. doi: 10.1097/MD.0000000000015584.
8. Shi JM, Meng XJ, Luo Y, et al. Clinical characteristics and outcome of isolated extramedullary relapse in acute leukemia after allogeneic stem cell transplantation: a single-center analysis. Leuk Res. 2013;37(4):372-377. doi: 10.1016/j.leukres.2012.12.002.
9. Mo XD, Kong J, Zhao T, et al. Extramedullary relapse of acute leukemia after haploidentical hematopoietic stem cell transplantation: incidence, risk factors, treatment, and clinical outcomes. Biol Blood Marrow Transplant. 2014;20(12):2023-2028. doi: 10.1016/j.bbmt.2014.08.023.
10. Harris AC, Kitko CL, Couriel DR, et al. Extramedullary relapse of acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Haematologica. 2013;98(2):179-184. doi: 10.3324/haematol.2012.073189.
11. Hamdi A, Mawad R, Bassett R, et al. Central nervous system relapse in adults with acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(11):1767-1771. doi: 10.1016/j.bbmt.2014.07.005.
12. Giralt SA, Champlin RE. Leukemia relapse after allogeneic bone marrow transplantation: a review. Blood. 1994;84(11):3603-3612.
13. Solh M, DeFor TE, Weisdorf DJ, Kaufman DS. Extramedullary relapse of acute myelogenous leukemia after allogeneic hematopoietic stem cell transplantation: better prognosis than systemic relapse. Biol Blood Marrow Transplant. 2012;18(1):106-112. doi: 10.1016/j.bbmt.2011.05.023.
14. Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371-4383. doi: 10.1182/blood-2008-03-077974.
15. Lee KH, Lee JH, Choi SJ, et al. Bone marrow vs extramedullary relapse of acute leukemia after allogeneic hematopoietic cell transplantation: risk factors and clinical course. Bone Marrow Transplant. 2003;32(8):835-842. doi: 10.1038/sj.bmt.1704223.
16. Clark WB, Strickland SA, Barrett AJ, Savani BN. Extramedullary relapses after allogeneic stem cell transplantation for acute myeloid leukemia and myelodysplastic syndrome. Haematologica. 2010;95(6):860-863.
17. Simpson DR, Nevill T, Shepherd JD, et al. High incidence of extramedullary relapse of AML after busulfan/cyclophosphamide conditioning and allogeneic stem cell transplantation. Bone Marrow Transplant. 1998;22(3):259-264. doi: 10.1038/sj.bmt.1701319.
