Current Treatment of Peripheral T-cell Lymphoma
This review article written by Robert Stuver, MD, et al, reviews current and available treatments for peripheral T-cell lymphoma.
ABSTRACT
The peripheral T-cell lymphomas (PTCLs) are a notoriously diverse family of non-Hodgkin lymphomas with generally aggressive biology. Clinical management is challenging given a largely inadequate literature base comprised of few randomized trials and heterogeneous observational reports. Herein, we provide an account of our practice in the treatment of the 3 most common nodal PTCLs: PTCL, not otherwise specified, angioimmunoblastic T-cell lymphoma, and anaplastic large cell lymphoma (ALCL). In the up-front setting, we employ anthracycline-based induction, with the incorporation of brentuximab vedotin for all those with ALCL and consideration in those with other CD30-expressing PTCLs based on improved progression-free and overall survival in the absence of additional toxicity in the ECHELON-2 trial. We strongly consider high-dose therapy with autologous stem cell rescue in first complete remission. In the relapsed or refractory (R/R) setting, we often look to clinical trials or choose from 4 FDA-approved single agents—belinostat, brentuximab vedotin, romidepsin, and pralatrexate—based on tumor phenotype and side-effect profiles. Our goal in the R/R setting is achievement of complete remission followed by allogeneic transplant with curative intent in appropriate candidates or long-term disease control in others. Numerous investigational agents are advancing through trials and have potential to alter standards of care in the near future.
Oncology (Williston Park). 2022;36(5):293-305.
DOI: 10.46883/2022.25920960
Introduction
The peripheral T-cell lymphomas (PTCLs) are derived from the malignant transformation of postthymic T lymphocytes.1 They are uncommon and markedly heterogeneous, representing about 10% to 15% of all non-Hodgkin lymphomas and encompassing 28 distinct entities with differing postulated normal counterparts (Table 1). Despite significant variation in pathology and presentation, the most common subtypes—PTCL, not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), and systemic anaplastic large cell lymphoma (sALCL)—tend to be treated similarly and are the focus of this review. AITL falls within the broader category of nodal lymphomas of T follicular helper (TFH) cell origin, which also includes follicular T-cell lymphoma (FTCL) and nodal PTCL with TFH phenotype, both of which are treated similarly to AITL. Other, less-common subtypes, such as adult T-cell leukemia/lymphoma (ATL), T-cell prolymphocytic leukemia, T-cell large granular lymphocytic leukemia, mycosis fungoides/Sézary syndrome, and extranodal natural killer (NK)/TCL, nasal type, have unique treatments and approaches and are reviewed elsewhere.2-7
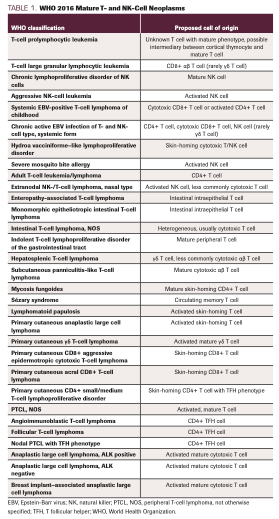
TABLE 1. WHO 2016 Mature T- and NK-Cell Neoplasms
Two large retrospective series are often quoted as historical controls in providing expected outcomes for patients with PTCL: the International T-cell Lymphoma Project (ITCLP) and the British Columbia Cancer Agency (BCCA) series. They report outcomes on 1314 and 199 cases, respectively.8,9 Most patients in these series were treated with up-front anthracycline-based induction without consolidative high-dose therapy and autologous stem cell rescue (HDT/ASCR). Five-year overall survival (OS) for the most common subtypes was generally less than 50%.8,9 However, we believe that current practices—namely, the addition of etoposide to induction regimens, incorporation of brentuximab vedotin (BV; Adcetris) in CD30-positive subtypes, offering consolidative HDT/ASCR to appropriate patients, and use of multiple approved agents in the relapsed/refractory (R/R) setting—likely result in outcomes that are superior to those in these historical data sets. Still, the treatment of PTCL is hindered by its rarity and subsequent lack of randomized data. As such, treatment decisions and strategies are founded upon our interpretation of the best existing data in concert with clinical experience and often significant joint decision-making with our patients. National guidelines exist from the National Comprehensive Cancer Network (NCCN)10 and the British Society for Haematology.11 In this review, we provide our approach to PTCL, highlighting what we consider “standard-of-care” practices and emphasizing ongoing attempts to increasingly personalize treatment for individual histologies and unique genetic subsets.
Current Standards for Diagnosis
A considerable challenge in managing PTCL is arriving at a confident diagnosis. TCLs are rare, and potential diagnoses should be approached accordingly. We recommend review by an experienced hematopathologist at a high-volume center whenever possible. Still, consensus may not be uniform. For example, in the ITCLP, a consensus diagnosis (agreement among 3 of 4 expert pathologists) was reached in only 74% to 81% of cases for anaplastic lymphoma kinase (ALK)–negative sALCL, PTCL-NOS, and AITL.8 Moreover, 10.4% of cases were either misdiagnosed initially or deemed unclassifiable upon expert review. In addition, clinical features, often unavailable to the pathologist, can influence diagnoses. For example, clinical input, such as presence of a rash, led to a change in diagnosis in 6.4% of all cases within the ITCLP. Knowledge of human T-cell leukemia virus (HTLV)–1 status led to 38.7% of cases of PTCL-NOS being reclassified to ATL. These findings underscore the potential importance of communication between clinicians and pathologists and thoughtful integration of the clinical presentation with histology, immunophenotyping, and molecular studies.
For initial diagnosis, excisional or incisional biopsy is preferred over core needle biopsy if feasible. Histologic review is mandatory. Cases classified as PTCL-NOS are those that cannot be classified as one of the specific TCLs in the World Health Organization classification system. PTCL-NOS has a broad histological presentation but in lymph node most commonly results in effacement of normal architecture with a diffuse infiltrate of medium- and/or large-sized cells with many mitotic figures. Some cases show a prominent inflammatory cell background.12 AITL characteristically displays a distinct clustering pattern of small- to medium-sized neoplastic lymphocytes with clear cytoplasm and minimal atypia near high endothelial venules (HEVs), progressively resulting in hyperplasia and arborization (branching out) of HEVs within the paracortex, hence the angioimmunoblastic name.12 The clusters are surrounded by an expanded follicular dendritic meshwork, and the background is typically rich in mixed inflammatory cells, including reactive lymphocytes, histiocytes, plasma cells, and eosinophils. Neoplastic cells are negative for Epstein-Barr virus (EBV), but an infiltrate of EBV-positive B cells is almost always present, at times progressing to advanced EBV-driven B-cell lymphoproliferative disorders, most commonly diffuse large B-cell lymphoma.13 Nodal PTCL with TFH phenotype is immunophenotypically similar to AITL but typically shows a diffuse infiltration pattern without prominent vascular proliferation, expansion of follicular dendritic cell meshworks, or a polymorphic inflammatory cell background. In ALK-positive sALCL, broad morphology is observed with multiple recognized histologic patterns, but there is universal detection of typically large, hallmark cells with eccentric, horseshoe-shaped nuclei and prominent Golgi zones.14 Other classic findings are multinucleated cells with nuclei in a wreath-like pattern and cells that appear to have nuclear inclusions (“doughnut cells”).15 The most common morphology, termed the common pattern, demonstrates cohesive sheets and clusters of mostly large cells with frequent hallmark cells that efface the lymph node architecture or grow within sinuses.15 ALK-negative sALCL shows morphology similar to the common pattern of ALK-positive sALCL, and they cannot be reliably distinguished from one another based on morphologic grounds alone.
Immunohistochemistry (IHC) studies are paramount in diagnosis and should include pan–B- and T-cell markers. In general, TCLs variably express T-cell antigens and lack B-cell antigens, although no one marker reliably or consistently defines individual subtypes. It is critical to recognize that unlike B-cell lymphomas, which are characterized by an algorithmic diagnostic approach with largely reproducible immunophenotypic patterns, TCLs display greater antigen aberrancy that vary within subtypes or even individual disease course.16,17 For example, in one immunophenotype review of 76 cases of PTCL, 82% displayed aberrant expression of 1 or more pan–T-cell markers.16 The majority of nodal PTCL-NOS cases express CD4 and lack CD8, although CD4-negative/CD8-positive, CD4-negative/CD8-negative, and CD4-positive/CD8-positive cases have all been documented.12,17 They typically express T-cell receptor beta (beta F1), frequently show loss of CD5 and CD7, and sometimes express cytotoxic markers (eg, TIA1, granzyme B, perforin); rarely, single TFH-cell markers may be observed.17 In contrast, the neoplastic cells in AITL are nearly always CD4-positive and defined by a TFH immunophenotype, including CD10, CXCL13, CXCR5, ICOS, BCL6, PD1 (CD279), CD200, SAP, and MAF, although the extent and intensity of staining is variable.18-24 Expression of at least 2, and preferably 3, TFH-cell markers in addition to CD4 is recommended to assign a TFH phenotype in a nodal TCL and to establish a diagnosis of nodal PTCL with TFH phenotype. By definition, CD30 is expressed in sALCL and is generally strongly and uniformly positive, but it can be diminished in the smaller cells seen in some of the uncommon histologic variants of ALK-positive sALCL (and seen variably in PTCL-NOS and AITL).25 Systemic ALCLs are CD4-positive in the majority of cases, show frequent loss of pan–T-cell antigens, and commonly express cytotoxic markers. Assessment of ALK rearrangement status is needed in sALCL, which is usually performed by IHC with anti-ALK monoclonal antibodies (ALK expression is absent in all normal postnatal human tissue except rare cells in the central nervous system).26 Cytogenetic or sequencing analyses can identify the ALK rearrangement but are not necessary if ALK staining is positive by IHC. Distinguishing ALK-negative sALCL from CD30-positive PTCL-NOS can be challenging, although the presence of hallmark cells, cohesive or sinusoidal growth, significant loss of T-cell markers, and strong, uniform CD30 positivity favor ALK-negative sALCL.1 Rare diagnostic challenges include cases with traditional B-cell marker positivity (such as CD20 or CD79a) or cases exhibiting co-expression of CD15 and CD30, which is typically associated with classic Hodgkin lymphoma.27-30 Therefore, whereas IHC is an essential component in diagnosis of all PTCLs, it must be supported by a comprehensive morphological and clinical evaluation.
Molecular Testing
We perform T-cell clonality testing by polymerase chain reaction or next-generation sequencing (NGS) in order to detect clonal T-cell receptor (TCR) gene rearrangements. A potential pitfall is equating the presence of clonality to a diagnosis of PTCL, yet clonal rearrangement alone is not sufficient for diagnosis. False positives can be seen and nonmalignant clones are not uncommon in reactive instances, such as infection or autoimmunity, in which an exaggerated immune response can result in T-cell clonal expansion.31,32 Moreover, the absence of TCR rearrangements does not exclude diagnoses, as amplification may fail in cases of poor sampling or low tumor burden. Rearrangement of the T-cell receptor γ gene (TRG) alone does not constitute a primary γδ TCL, which usually displays a uniqueγδT-cell phenotype and has additional diagnostic features.
Aside from TCR rearrangement testing, no molecular testing is absolutely mandatory in diagnosis or treatment selection. However, we attempt NGS when feasible in order to gain additional diagnostic and prognostic information. We use Memorial Sloan Kettering – Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT), a targeted panel utilizing hybridization capture–based NGS.33 Multiple publications now exist detailing the molecular landscape of various PTCL histologies, and with larger data sets and deeper depths of sequencing, the diagnostic and prognostic value of certain molecular alterations is increasingly noted. Most notable are the high frequencies of overlapping mutations in epigenetic modifiers in AITL.34-38 These include TET2 (50%-80%), DNMT3A (20%-30%), and IDH2 (20%-30%), especially IDH2 R172 mutations.38 The RHOA G17V mutation is also observed in AITL in up to 70% of cases.39,40 However, none of these mutations are entirely specific for AITL, as they can be observed in other entities, especially nodal PTCL with TFH phenotype. Other molecular aberrations that carry diagnostic value include ALK rearrangements in ALK-positive sALCL, rearrangement of DUSP22 or TP63 in ALK-negative sALCL,41 and ITK-SYK fusions in follicular TCL.42,43 With the exception of ALK, none are entirely pathognomonic.
Up-front strategies based on molecular profiles are not currently borne out. Recent data from our center show that TP53 aberrations may be associated with decreased responses and inferior survival outcomes in those treated with up-front anthracycline-based combination therapy.44 Various gene expression signatures may also delineate high-risk patients,45,46 although gene expression profiling is not routinely used or always readily available in clinical practice. Induction strategies based on mutational profiling are not defined, and much of the aforementioned data lack large-scale prospective validation. While we foresee decision-making based on tumor molecular profiling to play a key role in future treatment paradigms, this concept is not yet practiced.
Initial Work-up
Other testing that we routinely obtain at initial diagnosis includes a complete blood count, comprehensive metabolic panel, lactate dehydrogenase (LDH), and serology for HTLV-1 (with anti–HTVL-1 antibodies), which lends diagnostic support to ATL if positive. We obtain bone marrow aspirate and biopsy for full staging, as marrow involvement likely carries prognostic significance.47,48 Staging is by the Lugano Modification of Ann Arbor Staging System.49 For staging, an 18-fluoro-2-deoxyglucose PET/CT scan is recommended over CT, given the potential for extranodal disease. We prefer full body scanning (vertex to feet), given frequent cutaneous involvement.50 Nearly 30% of patients in our retrospective series had detectable disease by PET/CT that was not identified by standard CT of the neck, chest, abdomen, and pelvis.50 However, PET/CT imaging is not adequately sensitive at detecting bone marrow involvement in PTCL and does not replace bone marrow evaluation.51,52
Stratification for Treatment Selection
Whenever possible, the goal of initial therapy is cure or long-term remission. We estimate baseline prognosis with the International Prognostic Index (IPI), but scores do not influence our management and there is no well-defined paradigm for treatment selection by prognosis. Other prognostic scoring systems for PTCL have been proposed, but none provide practice-changing improvement over IPI.53 Molecular features, such as TP53 alterations44 and certain gene expression profiles,45,46 may prognosticate patients who have worse outcomes, although these are not currently factored into treatment algorithms. Essentially all patients require therapy, including those with limited-stage or low-risk disease by prognostic indices, as outcomes are still poor and the natural history is progressive disease. We offer participation in clinical trials to all eligible patients. Below we provide our “standard-of-care” approach in the up-front setting in the absence of a suitable clinical trial.
Therapy Selection
Anthracycline-Based Therapy
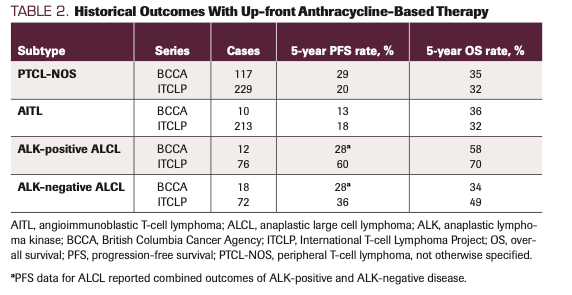
We consider CHOP-based therapy (cyclophosphamide, doxorubicin, vincristine, prednisone) as a standard of care in the up-front setting. Randomized trials of gemcitabine-based regimens54-56 and alternative cytotoxic platforms57 have not proven superior. The ITCLP and BCCA series serve as historical controls, providing the minimum expected outcomes when patients are given stand-alone anthracycline-based therapy without additional agents or consolidation (Table 2).8,9 Aside from ALK-positive sALCL, which consistently displays superior outcomes, 5-year progression-free survival (PFS) rates across these series were generally less than 30%, and 5-year OS rates were generally less than 40%. Despite poor survival outcomes, response rates to CHOP-based therapy are actually high. In the BCCA series, objective response rates (ORRs) for PTCL-NOS, AITL, and sALCL (both ALK-positive and ALK-negative) were 84%, 90%, and 76%, respectively. Contemporary reports show similarly high responses. For example, in ECHELON-2 (NCT01777152; see below), the randomized phase 3 trial of CHOP vs BV-CHP (BV plus cyclophosphamide, doxorubicin, and prednisone) in CD30-positive PTCLs, ORR in the CHOP-alone arm was 72%, with a complete response (CR) rate of 56%.58 Similarly, in the recently reported randomized phase 3 trial of CHOP vs romidepsin (Istodax) plus CHOP (NCT01796002), ORR in the CHOP-alone arm was 60%, with CR rate of 37%.59 Therefore, what we conclude from available data is that CHOP can provide an initial response for many patients, but fewer achieve CR and even fewer achieve durable responses. We employ 3 major strategies to improve response rate and durability: (1) addition of etoposide to CHOP in fit patients; (2) addition of BV in CD30-positive histologies; and (3) strong consideration for consolidative HDT/ASCR in patients achieving an initial response. Each of these strategies is detailed below.
TABLE 2. Historical Outcomes With Up-front Anthracycline-Based Therapy
Etoposide
Etoposide is a potent anti-lymphoma agent for which there are data to support its addition to CHOP (CHOEP) in the first-line setting. The NHL-B1 trial, conducted by the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL), was a randomized trial of CHOP-21 vs CHOEP-21 (and CHOP-14 vs CHOEP-14) in patients 60 years or younger with aggressive B- and T-cell lymphomas (notably, patients were excluded if LDH was elevated).60 The study met its primary end point: Five-year event-free survival (EFS) rates were significantly improved with the addition of etoposide to CHOP (69.2% for CHOEP-14/21 vs 57.6% for CHOP-14/21; P = .004). The CR rate was significantly improved as well (87.6% vs 79.4%; P = .003). Over 85% of patients in this study had B-cell lymphoma, but in a separate analysis of only those with TCL, the 3-year EFS rate remained significantly improved in the CHOEP group (75.4% vs 51.0%; P = .003).61
This finding was substantiated in a combined analysis of 3 DSHNHL trials comparing the addition of etoposide to CHOP in patients with TCL (3-year EFS rate, 70.5% after CHOEP vs 51.0% after CHOP; P = .004).61 Importantly, EFS significance was lost when patients with ALK-positive sALCL were excluded (P = .057) and EFS benefits did not translate to significant OS benefits (81.3% vs 75.2%; P = .285). In addition, etoposide adds considerable toxicity, particularly in those 60 years or older. Still, additional support for the incorporation of etoposide to CHOP-based therapy is seen in a phase 2 trial of dose-adjusted-EPOCH in 41 untreated patients with TCL, primarily PTCL-NOS and AITL (n = 38).62 The ORR was 78.0%, with a CR rate of 61.0%. Patients 60 years or younger had significantly improved ORR vs those older than 60 years (94.1% vs 66.7%; P = .036). The 2-year PFS and OS rates for all-comers were 73.2% and 53.3%, respectively, and again were improved in the younger population (PFS, 82.4%; OS, 62.5%). In contrast to the DSHNHL studies mentioned above, this study was enriched for poor-risk disease: 80.5% of patients had stage III/IV disease, 68.3% had elevated LDH, and 51.2% had IPI of 3 or greater. In addition, no patients received HDT/ASCR, speaking to the potential durability of this regimen even in a high-risk population, although follow-up was limited.
Our takeaway from the above data is that etoposide may be added to CHOP if it can be done so without significant excess toxicity. This applies primarily to patients with CD30 expression less than 10%, as we generally prefer BV-CHP in patients with 10% or greater expression; this is based on potentially increased efficacy of BV-CHP in the absence of significant toxicity, as demonstrated in ECHELON-2 (see below).58 Importantly, a recently reported phase 2 trial of BV-CHEP (BV plus cyclophosphamide, doxorubicin, etoposide, and prednisone) in untreated CD30-positive (≥1%) disease showed impressive ORR and CR rates of 91% and 80%, respectively.63 Consolidation with HDT/ASCR was permitted, as was BV consolidation for up to 10 additional cycles. The most frequent adverse events (AEs) included fatigue (73%) and peripheral sensory neuropathy (67%), with grade 3 neutropenia and febrile neutropenia occurring in 37.5% and 23%, respectively, despite mandatory granulocyte colony stimulating factor.63 Longer follow-up will dictate whether this regimen should supplant BV-CHP in CD30-positive histologies, although we will consider this regimen moving forward in individual cases when a maximal induction approach is desired. Finally, in those with at least 1% but less than 10% expression of CD30, there have been no prospective comparisons of BV-containing regimens vs non–BV-containing regimens, although a study investigating BV-CHP in treatment-naïve PTCL with less than 10% CD30 expression is ongoing (NCT04569032).
Brentuximab Vedotin
CD30 is universally expressed in sALCL and variably expressed across other nodal histologies, including PTCL-NOS and AITL.25,64 BV is an antibody-drug conjugate composed of an anti-CD30 monoclonal antibody linked to monomethyl auristatin E, a microtubule-disrupting agent. Based on encouraging results from a phase 2 trial of BV in R/R sALCL65 and a phase 1 trial of BV-CHP in CD30-positive PTCLs,66 the randomized, placebo-controlled, double-blind, phase 3 ECHELON-2 trial of BV-CHP vs CHOP was conducted.58 Eligible patients were adults with untreated PTCL with at least 10% CD30 expression. Histologies were limited to ALK-negative sALCL (n = 218; 48%), PTCL-NOS (n = 72; 16%), and AITL (n = 54; 12%), as well as ATL (n = 7; 2%), enteropathy-associated TCL (n = 3; 1%), and hepatosplenic TCL (n = 0; 0%). ALK-positive ALCL (n = 98; 22%) inclusion was limited to those with IPI of 2 or greater given superior outcomes in ALK-positive ALCL. A total of 452 patients underwent 1:1 randomization. The trial met its primary end point of PFS, with an impressive 48.2-month median PFS for BV-CHP vs 20.8 months for CHOP (HR, 0.71; 95% CI, 0.54-0.93; P = .011). The 75th-percentile OS was not reached for BV-CHP but was only 17.5 months for CHOP, translating to a 34% lower risk of death with BV-CHP. In addition, ORR (83% vs 72%) and CR (68% vs 56%) were improved with BV-CHP, notably without an appreciable increase in the incidence or severity of treatment-emergent AEs. The 5-year updated series show continued PFS and OS benefits.67 At a median follow-up of 44.3 months, median PFS for BV-CHP was 63.5 months vs 23.8 months for CHOP (HR for PFS, 0.70; 95% CI, 0.53-0.91; P = .0075). Median OS was not reached in either arm, and the estimated 5-year OS rate was 68.7% for BV-CHP vs 60.3% for CHOP (HR for OS, 0.74; 95% CI, 0.54-1.02; P = .0688). BV-CHP is the first and only regimen to show a survival advantage over an established therapy in PTCL, and based on these results, BV-CHP was approved by the FDA for previously untreated sALCL and other CD30-expressing PTCLs.
There are important caveats in regard to ECHELON-2. First, the trial population included only those with CD30 expression of 10% or greater. Therefore, in those with CD30 expression of 1% to 9%, the benefit of BV-CHP over CHOP is unproven (although CD30 expression level has not been shown to predict response).68 As mentioned above, an ongoing trial (NCT04569032) of BV-CHP in treatment-naïve PTCL with less than 10% CD30 expression is evaluating this question. In addition, the trial was enriched for sALCL given uniform CD30 positivity. As such, results were not powered to show superiority in subtypes other than sALCL; therefore, while we strongly consider BV-CHP in CD30-positive PTCL-NOS and AITL, subgroup analyses for PFS within these subtypes were not powered to detect significance (PTCL-NOS: HR, 0.79; 95% CI, 0.43-1.43; AITL: HR, 1.41; 95% CI, 0.64-3.11). Finally, the role of HDT/ASCR (see below) after BV-CHP is unclear. While ECHELON-2 allowed HDT/ASCR, the study was not designed to prospectively analyze outcomes in those who were and were not consolidated. In an exploratory analysis of only those achieving a CR in the BV-CHP arm, the 3-year PFS rate was improved in those who underwent consolidative HDT/ASCR vs those who did not (76.1% vs 53.3%; HR, 0.38; 95% CI, 0.18-0.82), but this population was small.69 With these caveats, we use BV-CHP as first-line therapy in all patients with sALCL, and we strongly consider it for CD30-positive PTCL-NOS and CD30-positive AITL. As noted above, the etoposide-containing regimen BV-CHEP appears effective, and we would consider this regimen in individual cases when a maximal induction approach is desired.63
High-Dose Chemotherapy With Autologous Stem Cell Rescue
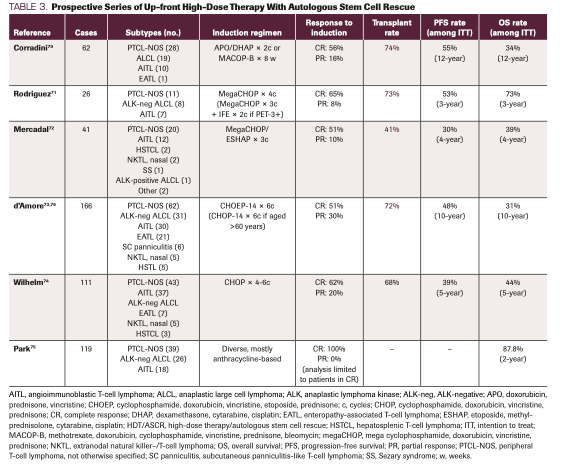
There is currently no consensus on the role of up-front HDT/ASCR, and no randomized comparisons have evaluated HDT/ASCR vs observation for those in first remission. Moreover, while multiple prospective observational series have been reported (Table 3), drawing definitive conclusions is challenging given heterogeneous populations and induction regimens, varying transplantation rates among intent-to-treat (ITT) populations, and differing rates of remission at the time of transplant.70-75 Still, based on these data, especially the prospective Nordic study (see below) and our own institutional experience, we favor HDT/ASCR with the goal to augment cure rate, but we do so in a tailored fashion only in patients who have achieved a CR to induction therapy (see Response Assessment below).
TABLE 3. Prospective Series of Up-front High-Dose Therapy With Autologous Stem Cell Rescue
The largest prospective trial of HDT/ASCR in PTCL, conducted by the Nordic Lymphoma Group, included 166 patients aged 18 to 67 years with newly diagnosed systemic PTCL between 2001 and 2007.73 Most patients had advanced-stage, intermediate- to high-risk disease (81% stage ≥III; 72% IPI ≥2). Induction was with CHOEP-14 for 6 cycles if patients were 60 years or younger and CHOP-14 for 6 cycles if older than 60 years. Response was measured by CT assessment, and patients in CR or partial response (PR) after induction underwent HDT/ASCR with BEAM conditioning (carmustine, etoposide, cytarabine, melphalan). Of 156 evaluable patients, 82% (n = 131) achieved CR or unconfirmed CR (CRu) (51%) or PR (30%). Of these 131 patients, 115 underwent HDT/ASCR, with the major reason for not reaching HDT/ASCR being infection (n = 5). Among the ITT population, 5-year PFS and OS rates were 44% and 51%, respectively. Patients with ALCL outperformed those with PTCL-NOS and AITL (ALCL: PFS, 61%; OS, 70%; PTCL-NOS: PFS, 47%; OS, 52%; AITL: PFS, 38%; OS, 47%). A multivariate analysis showed age, performance status of 2 or more, and bone marrow involvement to be negative prognostic factors, whereas female sex and ALCL histology were associated with improved survival outcomes. Ten-year follow-up showed persistent benefit, with 10-year PFS and OS rates among the ITT cohort of 48% and 31%, respectively.76 ALCL (PFS, 48%; OS, 48%) continued to outperform PTCL-NOS and AITL at 10 years.
Multiple other observational series exist.70-81 Heterogeneity precludes definitive conclusions, but a somewhat consistent finding is that consolidation is most effective in patients who are more chemosensitive, as evidenced by achieving CR in the frontline setting. The most compelling example comes from the Comprehensive Oncology Measures for Peripheral T-cell Lymphoma Treatment (COMPLETE) study, a large prospective cohort study of US patients with newly diagnosed PTCL.75 This report compared outcomes among patients with PTCL-NOS, AITL, and ALK-negative sALCL in first CR (CR1) who underwent HDT/ASCR (n = 36) vs observation (n = 83). Median PFS for those who underwent HDT/ASCR was superior to those who were observed (57.6 months vs 47.5 months; P = .23), as was the estimated 2-year OS rate (87.8% vs 70.2%; P = .06), although neither difference was statistically significant. Among those with AITL, survival was notably increased in the HDT/ASCR vs non-HDT/ASCR group (2-year PFS rate, not reached vs 18.6 months; P = .10; 2-year OS rate, not reached vs 24.3 months; P <.01). For ALK-negative sALCL and PTCL-NOS, the HDT/ASCR cohorts experienced nonsignificant survival improvements. Univariate analyses showed significantly improved 2-year OS rates for those with advanced-stage disease (87.2% vs 58.4%; P = .013) and intermediate to high IPI score (87.6% vs 59.2%; P = 0.018) who underwent HDT/ASCR vs those who did not. In a multivariate analysis, HDT/ASCR was associated with superior OS with an HR of 0.37 (95% CI, 0.15-0.89; P = .03), regardless of stage or IPI.
Our takeaway from the above study is that while there was no statistically significant difference in survival between the HDT/ASCR group and non–HDT/ASCR group en masse, those with AITL and/or those with advanced-stage, high-risk disease may benefit most from consolidation in CR1. In practice, based on our experience and the above data, we strongly consider HDT/ASCR in all patients with PTCL-NOS, AITL, and ALK-negative sALCL who achieve CR1, but we have a nuanced, detailed discussion that heavily incorporates patient preferences. A prior retrospective review of 34 patients at our institution who underwent HDT/ASCR in CR1 showed 4-year PFS and OS rates of 54.9% and 67.4%, respectively.80
An important, emerging caveat regards patients with ALK-negative sALCL with DUSP22 chromosomal rearrangement. DUSP22 rearrangements—which
result in decreased expression of dual-specificity phosphatase-22, an enzyme that regulates mitogen-activated protein kinase signaling—have been reported in about 30% of ALK-negative sALCL.82,83 Limited reports show improved survival for DUSP22-rearranged sALCL over those with TP63-rearranged or triple-negative disease (defined as lacking ALK, DUSP22, and TP63 rearrangements).41,82 A combined analysis of 3 published series84 evaluating the impact of up-front HDT/ASCR on survival by ALK, DUSP22, and TP63 found similar 5-year OS in those with DUSP22-rearranged sALCL who underwent HDT/ASCR vs those who did not. However, overall numbers in this report are small, no data have been confirmed prospectively, and other reports are conflicting.85 In practice, while we assess DUSP22 status, we interpret the above data with extreme caution when dealing with individual patients and do not use it as a sole variable in transplant calculations.
Finally, we generally do not offer up-front allogeneic transplant to those in CR1 with PTCL-NOS, AITL, and ALK-negative sALCL (unlike other
histologies, such as ATL and hepatosplenic TCL). A recently reported randomized phase 3 trial of HDT/ASCR vs allogeneic transplant as first-line therapy in poor-risk PTCL (defined as stage II-IV and age-adjusted IPI >0) showed no statistical difference in the primary end point of 3-year EFS rate (43% after allogeneic transplant vs 38% for HDT/ASCR).86 The trial ended early for futility. In those who reached consolidation, relapse rate in the allogeneic transplant group was 0% vs 36%, but any graft-vs-lymphoma effect was counterbalanced by transplant-related mortality (31% after allogeneic transplant vs 0% for HDT/ASCR). Whether high-risk clinical or molecular groups may benefit more from allogeneic transplant in CR1 requires ongoing research.
Response Assessment
In the absence of new symptoms or clinical concerns, we typically perform first response assessment after 4 cycles of induction therapy with integrated PET/CT. Response evaluation is by Lugano criteria.49,87 In those with an objective response, we complete planned induction therapy and repeat PET/ CT for final response evaluation. For those with bone marrow involvement at initial staging, we repeat bone marrow assessment. Interim PET (iPET) after 4 cycles using a 5-point score (5PS) is prognostic, as shown in a retrospective study performed at our center of 112 patients with PTCL treated with up-front anthracycline-based therapy with intent to consolidate with HDT/ASCR.88 Interim 5PS of 4 or 5 (uptake above liver) predicted worse OS (HR, 11.025; 95% CI, 4.409-27.571; P <.001) and worse EFS (HR, 3.573; 95% CI, 1.824-6.997; P <.001). When combined with prognostic scoring systems, such as IPI and Prognostic Index for T-cell Lymphoma (PIT), iPET scan is strikingly prognostic. In those with iPET 5PS score of 1 to 3 and PIT score of 1 or lower (on a scale of 0-4, 4 indicating worse prognosis), 4-year OS and EFS rates were 85% and 62%, respectively, compared with 4-year OS and EFS rates of 0% in those with iPET 5PS of 4 or 5 and PIT of 2 or higher. Thus, we use interim evaluation to identify patients at risk high risk for poor outcomes, and we consider an early alteration of therapy in these patients as they may be demonstrating chemoresistance. Outside of a clinical trial, we do not yet employ routine minimal residual disease (MRD) testing, but a clinical trial evaluating the prognostic value of T-cell receptor clonotyping is ongoing (NCT03297697).89 We envision MRD testing to possibly play an increased role in the early identification of patients who will not be cured with combination chemotherapy approaches and require alternative approaches.
Relapsed and Refractory Disease
Historic outcomes for patients with R/R disease are extremely poor. In this setting, median PFS and OS in the absence of allogeneic transplant are
3.1 months and 5.5 months, respectively.90 The only reliably curative approach is salvage therapy followed by allogeneic transplant, but achieving a second objective response is challenging, allogeneic transplant carries significant morbidity and mortality, and many patients at this point in their disease course have become physiologically ineligible for intensive therapy.
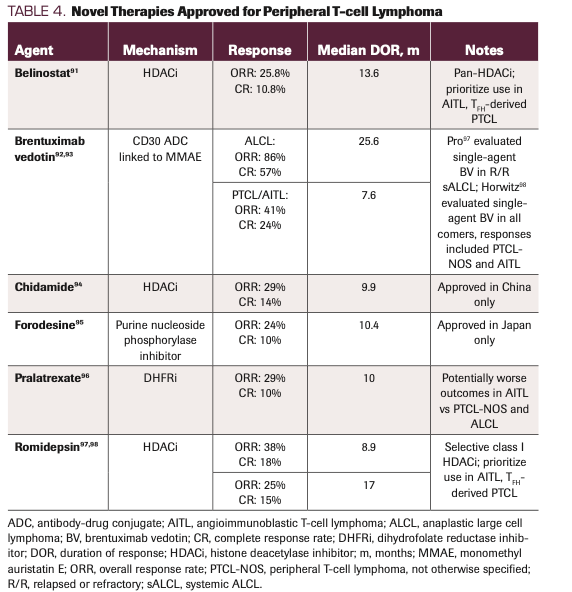
For reinduction, we attempt clinical trial enrollment whenever available. Otherwise, there are currently 4 approved therapies for R/R disease: belinostat, BV, pralatrexate, and romidepsin (see comment on withdrawal below) (Table 4). With the exception of BV in CD30-positive disease, these agents were generally approved based on ORRs of less than 30%.91-98 BV was initially approved for relapsed sALCL based on a phase 2 trial of BV in R/R ALCL, showing ORR of 86% and CR of 57%.92 A phase 2 trial of BV in R/R CD30-positive non-Hodgkin lymphomas, including PTCL, showed activity in PTCL-NOS (ORR, 33%; CR, 14%) and AITL (ORR, 54%; CR, 38%) as well.93
TABLE 4. Novel Therapies Approved for Peripheral T-cell Lymphoma
Two histone deacetylase (HDAC) inhibitors have been approved in the R/R setting. Romidepsin, a class I selective HDAC inhibitor, was approved in 2011 based on a phase 2 trial showing ORR of 25% and CR/CRu rate of 15% in 131 patients with R/R PTCL. In August 2021, accelerated approval of romidepsin was withdrawn by Bristol Myers Squibb based on a negative up-front trial of CHOP vs romidepsin plus CHOP, although the agent is still available for use. Belinostat, a broad HDAC inhibitor, was approved in 2014 based on the phase 2 BELIEF trial, which showed an ORR of 25% and a CR rate of 11% in
129 patients.91 While initial reports did not find marked differences in response rates among subtypes, there is an increasing recognition of differential sensitivity to HDAC inhibitors in those with a TFH phenotype, including AITL and nodal PTCL with TFH phenotype. These histologies are enriched for somatic mutations in proteins involved in epigenetic regulation, such as DNMT3A, IDH2, and TET2, likely conferring enhanced vulnerability to HDAC inhibition.37 A recently reported multi-institutional retrospective effort of 164 patients treated with an HDAC inhibitor in the R/R setting showed an ORR of 56.5% in those with TFH phenotype vs 29.4% in those with non-TFH phenotype (P = .003).99 Logistic regression found TFH phenotype to be significantly associated with objective response (HR, 0.322; P = .009), and certain mutational signatures typical of TFH phenotype (TET2 and/or DNMT3A and/or RHOA) were significantly more common in responders than nonresponders (83% vs 40%; P = .034). We heavily consider TFH phenotype and mutational profile in considering second-line agents, prioritizing HDAC inhibitor use in this population.
The final approved agent, pralatrexate, is a dihydrofolate reductase inhibitor with high affinity for reduced folate carrier–type 1, an oncofetal protein. Pralatrexate was evaluated in the phase 2 PROPEL trial (NCT00364923).96 In this trial of 115 heavily pretreated patients (median of 3 prior systemic therapies; 24% had never responded to therapy; 16% had received prior HDT/ASCR), ORR was 29% with a CR rate of 11%. Although not powered to detect differences among subhistologies, patients with AITL did noticeably worse (ORR, 8%) than those with PTCL-NOS (ORR, 32%) and ALCL (ORR, 35%). Myelosuppression and mucositis are common, and we use folic acid and B12 supplementation in all patients and consider adjunctive leucovorin in patients with considerable mucosal toxicity.100
Combination chemotherapy can be considered in the R/R setting, although data are sparse and regimens are borrowed from salvage strategies in B-cell lymphoma. Regimens include ifosfamide, carboplatin, etoposide (ICE),101 dexamethasone, cytarabine, cisplatin (DHAP),102 and gemcitabine, dexamethasone, cisplatin (GDP).103 Additional agents with activity include alemtuzumab,104 bendamustine,105 bortezomib (Velcade),106 cyclosporine (in AITL),107 gemcitabine,108 and lenalidomide (Revlimid).109 Some reports show survival benefits in patients treated with noncytotoxic therapy over chemotherapy in the R/R setting,110,111 but these are severely limited by observational design, and no formal comparison between any of the above approved single agents vs second-line combination chemotherapy has occurred.
For fit patients, the goal of each of the above strategies is reduction in disease burden followed by allogeneic transplant with reduced intensity/nonmyeloablative conditioning.112 In the largest series to date of allogeneic transplant of more than 500 patients across 12 institutions, 5-year PFS and OS rates among all subtypes were 39.4% and 50.8%, respectively.113 No other therapy can offer this degree of prolonged survival, and without transplant, most patients die of disease.114 Patients with cutaneous TCL had noticeably worse sensitivity to transplant in this series, with 5-year PFS rates of just 18.6%, although 5-year OS (44.0%) was similar to that of the entire cohort. When compared specifically with PTCL-NOS or sALCL (both ALK-positive and ALK-negative), AITL had nonsignificant improvements in median 5-year PFS (51.4 months vs 18.4 months; P = .14) and OS (not reached vs 73.1 months; P = .26). A recently reported meta-analysis of allogeneic transplant in R/R PTCL reported similar findings, evaluating 30 trials with more than 800 patients, showing 5-year PFS and OS rates of 48% and 54%, respectively.115 Therefore, we provide early referral to a transplant specialist for every patient who is beginning second-line (or later) curative-intent treatment, especially with the increasing use and comparable outcomes of haploidentical donor transplant for those lacking HLA-matched donors.116-118
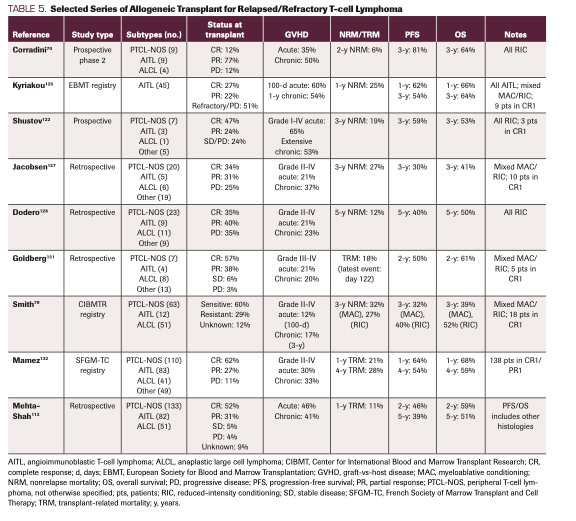
Multiple other observational series exist as well (Table 5).79,113,119-133 A unifying observation from each of these studies is that disease status at the time of the transplant is a critical prognostic factor. In the largest series to date, median PFS for those in CR at transplant was significantly greater than those in PR (44.6 months vs 8.6 months; P <.001).113 For those with progressive disease, PFS was a mere 3.5 months.113 Therefore, we are hesitant to transplant any patient with existing disease given these significantly worse outcomes, and we generally prefer further attempts to achieve CR. For those who relapse after allogeneic transplant, survival is generally less than 1 year.113 Only sporadic long-term remissions can be achieved with donor lymphocyte infusion or immunosuppression withdrawal.128,134,135
TABLE 5. Selected Series of Allogeneic Transplant for Relapsed/Refractory T-cell Lymphoma
Emerging Novel Therapies
A plethora of promising small molecule inhibitors are beginning to capitalize on targeted inhibition of vulnerable oncogenic pathways and intracellular processes. Particularly encouraging agents include duvelisib (Copiktra), cerdulatinib, ruxolitinib (Jakafi), tipifarnib, valemetostat, and ALK inhibitors, among others. Duvelisib is an oral inhibitor of the γδ isoform of PI3K, an enzyme family critical in cellular signaling through the PI3K-Akt-mTOR pathway. Completed phase 1 and ongoing phase 2 evaluations of duvelisib show consistent response rates in R/R PTCL, with an ORR of 50% and CR rate of 32.1% in the phase 2 dose-expansion cohort.136-138 Duvelisib is now listed by NCCN as a second-line agent. In the up-front setting, a recently initiated Alliance/Intergroup (NCT04803201) trial is randomizing patients with untreated CD30-negative (<10%) PTCL to duvelisib plus CHO(E)P vs azacytidine plus CHO(E)P vs CHO(E)P, in attempts to move duvelisib (or azacytidine) forward in therapy (CHOEP in those 60 years and younger and CHOP in all others). Cerdulatinib is a pan-JAK/SYK pathway inhibitor with an ORR of 35% in R/R PTCL, with notable phase 2 activity in AITL and nodal PTCL with TFH phenotype (ORR, 55%).139 Ruxolitinib, an oral selective JAK1/2 inhibitor, capitalizes on frequent overactivation of the JAK/STAT pathway in TCLs140 and has promising results in the R/R setting, especially in those with activating JAK/STAT mutations or elevated phosphorylated STAT3 expression (vs phosphorylated S6, which may predict lack of response).141 Tipifarnib is a potent oral inhibitor of farnesyltransferase that results in decreased secretion of CXCL12, a chemokine responsible for T-cell homing and maintenance of immune cell progenitors. Tipifarnib is particularly active in AITL and PTCL-NOS with CXCL12 genotype.142 Valemetostat, a potent oral dual inhibitor of enhancer of zeste homologue (EZH) 1 and 2, has broad activity against B-cell lymphomas and TCLs, and it has particularly encouraging activity in AITL and ATL, with an ORR near 50%.143,144 The pivotal phase 2 VALENTINE-PTCL01 trial (NCT04703192) of valemetostat in R/R PTCL is enrolling. In young adults, crizotinib (Xalkori) has recently gained FDA approval for R/R ALK-positive sALCL, demonstrating an ORR of 88%, with a CR rate of 81%, in 23 patients aged at least 21 years.145 Another ALK inhibitor, alectinib (Alecensa), has recently been NCCN-listed as second-line therapy in ALK-positive sALCL.146 Novel-novel combinatorial approaches involving many of the aforementioned agents, such as romidepsin plus duvelisb147 and romidepsin plus lenalidomide,148 have proven activity as well. Strategies to move novel-novel platforms further in therapy are warranted.
Cellular therapies for TCLs are in their infancy, but multiple products are under investigation. These include antibody therapies, such as the innate bispecific CD16/CD30 cell engager AFM13 (REDIRECT; NCT04101331), the anti-CD47 antibody magrolimab (including in combination with mogamulizumab; NCT04541017), and chimeric antigen receptor (CAR) T-cell therapy. Given the attractiveness of CD30 as a target, investigations of CD30-directed autologous CAR T cells are ongoing, although these trials have primarily focused on Hodgkin lymphoma.149,150 CD30-directed CAR T cells coexpressing CCR4 are also under investigation (NCT03602157), although only 2 patients with cutaneous TCL have reported outcomes.151 CD5 is an additional attractive target, and autologous CD5 CAR T cells are being explored as a bridge to allogeneic transplant in patients with R/R mature TCLs (NCT03081910).152 Preliminary results show bridging ability without severe infections or safety concerns.152 An additional trial of a CD70-directed allogeneic CAR T cell in patients with R/R mature TCLs is ongoing (COBALTY-LYM; NCT04502446). It is too early to discern the role of cellular therapies in TCLs.
Conclusions
Despite major advances in the treatment of hematologic malignancies over the last decade, the treatment of PTCL remains a distinct challenge, owing to its aggressive biology and limited clinical trial literature. Our treatment begins with a firm diagnostic evaluation rooted in expert hematopathology review. In the up-front setting, we use BV-CHP in sALCL and prefer BV-CHP in CD30-positive PTCL-NOS and AITL, although we recognize subtype-specific differences have emerged with extended ECHELON-2 data. For CD30-negative disease, we use standard CHOP and strongly consider CHOEP for those in whom we desire maximal chemotherapy induction. We strongly consider HDT/ASCR for those in first complete remission, regardless of induction therapy. In the R/R setting, we prefer clinical trial enrollment; when unavailable, we utilize approved single-agent therapy or salvage combination chemotherapy, with particular preference for HDAC inhibition for nodal lymphomas of TFH cell origin, including AITL. Our goal is remission followed by allogeneic transplant. At any time point, we prefer clinical trial enrollment for eligible patients. Promising investigative agents are on or just beyond the horizon, and we are hopeful for multiple regulatory approvals in the coming years that extend survival and change the current treatment outlined above.
Disclosure: RS, ZDEP, WTJ, NL have no disclosures. NK has research funding from Seattle Genetics. AJM has received research support from ADC Therapeutics, Beigene, Miragen, Seattle Genetics, Merck, Bristol-Myers Squibb, Incyte, and SecuraBio. AJM has received honoraria from Imbrium Therapeutics L.P./Purdue, Janpix Ltd., Merck, Seattle Genetics, and Takeda. CSS has served as a paid consultant on advisory boards for: Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite/a Gilead Company, Celgene/BMS, Gamida Cell, Karyopharm Therapeutics and GSK. CSS has received research funds for clinical trials from: Juno Therapeutics, Celgene/BMS, Bristol-Myers Squibb, Precision Biosciences, Actinium Pharmaceuticals and Sanofi-Genzyme. SMH has consulted, received honorarium from, or participated in advisory boards for Acrotech Biopharma, ADC Therapeutics, Astex, Merck, C4 Therapeutics, Celgene, Cimieo Therapeutics, Daiichi Sankyo, Janssen, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Seattle Genetics, Shoreline Biosciences, Inc, Takeda, Trillium Therapeutics, Tubulis, Verastem/SecuraBio, and Vividion Therapeutics.
Author Affiliations:
Robert Stuver, MD1; Zachary D. Epstein-Peterson, MD1; William T. Johnson, DO1; Niloufer Khan, MD, MS1; Natasha Lewis, MD2; Alison J. Moskowitz, MD1; Craig S. Sauter, MD1; Steven M. Horwitz, MD1
1Lymphoma Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY.
2Hematopathology Service, Department of Pathology and Laboratory Medicine, Memorial Sloan Kettering Cancer Center, New York, NY.
References
- Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC Press; 2017.
- Cook LB, Phillips AA. How I treat adult T-cell leukemia/lymphoma. Blood. 2021;137(4):459-470. doi:10.1182/blood.2019004045
- Braun T, von Jan J, Wahnschaffe L, Herling M. Advances and perspectives in the treatment of T-PLL. Curr Hematol Malig Rep. 2020;15(2):113-124. doi:10.1007/s11899-020-00566-5
- Lamy T, Moignet A, Loughran TP Jr. LGL leukemia: from pathogenesis to treatment. Blood. 2017;129(9):1082-1094. doi:10.1182/blood-2016-08-692590
- Whittaker S, Hoppe R, Prince HM. How I treat mycosis fungoides and Sézary syndrome. Blood. 2016;127(25):3142-3153. doi:10.1182/blood-2015-12-611830
- Tse E, Kwong Y-L. How I treat NK/T-cell lymphomas. Blood. 2013;121(25):4997-5005. doi:10.1182/blood-2013-01-453233
- Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood. 2018;131(23):2528-2540. doi:10.1182/blood-2017-12-791418
- Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130. doi:10.1200/JCO.2008.16.4558
- Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann. Oncol. 2004;15(10):1467-1475. doi:10.1093/annonc/mdh392
- NCCN. Clinical Practice Guidelines in Oncology. T-cell lymphomas, version 1.2021. Accessed 1 March 2022. https://www.nccn.org/progessionals/physician_gls/pdf/t-cell.pdf.
- Fox CP, Ahearne MJ, Pettengell R, et al. Guidelines for the management of mature T‐ and natural killer‐cell lymphomas (excluding cutaneous T‐cell lymphoma): a British Society for Haematology guideline. Br J Haematol. 2022;196(3):507-522. doi: 10.1111/bjh.17951
- Jaffe ES. Pathobiology of peripheral T-cell lymphomas. Hematology Am Soc Hematol Educ Program. 2006:317-322. doi:10.1182/asheducation-2006.1.317
- Zettl A, Lee S-S, Rüdiger T, et al. Epstein-Barr virus-associated B-cell lymphoproliferative disorders in angloimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified. Am J Clin Pathol. 2002;117(3):368-379. doi:10.1309/6UTX-GVC0-12ND-JJEU
- Amador C, Feldman AL. How I diagnose anaplastic large cell lymphoma. Am J Clin Pathol. 2021;155(4):479-497. doi: 10.1093/ajcp/aqab012
- Jaffe ES. Anaplastic large cell lymphoma: the shifting sands of diagnostic hematopathology. Mod Pathol. 2001;14(3):219-228. doi:10.1038/modpathol.3880289
- Hastrup N, Ralfkiaer E, Pallesen G. Aberrant phenotypes in peripheral T cell lymphomas. J Clin Pathol. 1989;42(4):398-402. doi:10.1136/jcp.42.4.398
- Went P, Agostinelli C, Gallamini A, et al. Marker expression in peripheral T-cell lymphoma: a proposed clinical-pathologic prognostic score. J Clin Oncol. 2006;24(16):2472-2479. doi:10.1200/JCO.2005.03.6327
- Roncador G, García Verdes-Montenegro J-F, Tedoldi S, et al. Expression of two markers of germinal center T cells (SAP and PD-1) in angioimmunoblastic T-cell lymphoma. Haematologica. 2007;92(8):1059-1066. doi:10.3324/haematol.10864
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95(3):432-439. doi:10.3324/haematol.2009.010991
- Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30(7):802-810. doi:10.1097/01.pas.0000209855.28282.ce
- de Leval L, Rickman DS, Thielen C, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109(11):4952-4963. doi:10.1182/blood-2006-10-055145
- Grogg KL, Attygalle AD, Macon WR, Remstein ED, Kurtin PJ, Dogan A. Expression of CXCL13, a chemokine highly upregulated in germinal center T-helper cells, distinguishes angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified. Mod Pathol. 2006;19(8):1101-1107. doi:10.1038/modpathol.3800625
- Dupuis J, Boye K, Martin N, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper T cells. Am J Surg Pathol. 2006;30(4):490-494. doi:10.1097/00000478-200604000-00009
- Dobay MP, Lemonnier F, Missiaglia E, et al. Integrative clinicopathological and molecular analyses of angioimmunoblastic T-cell lymphoma and other nodal lymphomas of follicular helper T-cell origin. Haematologica. 2017;102(4):e148-e151. doi:10.3324/haematol.2016.158428
- Sabattini E, Pizzi M, Tabanelli V, et al. CD30 expression in peripheral T-cell lymphomas. Haematologica. 2013;98(8):e81-e82. doi:10.3324/haematol.2013.084913
- Pulford K, Lamant L, Morris SW, et al. Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood. 1997;89(4):1394-1404.
- Quintanilla-Martinez L, Preffer F, Rubin D, Ferry JA, Harris NL. CD20+ T-cell lymphoma. neoplastic transformation of a normal T-cell subset. Am J Clin Pathol. 1994;102(4):483-489. doi:10.1093/ajcp/102.4.483
- Yao X, Teruya-Feldstein J, Raffeld M, Sorbara L, Jaffe ES. Peripheral T-cell lymphoma with aberrant expression of CD79a and CD20: a diagnostic pitfall. Mod Pathol. 2001;14(2):105-110. doi:10.1038/modpathol.3880265
- Blakolmer K, Vesely M, Kummer JA, Jurecka W, Mannhalter C, Chott A. Immunoreactivity of B-cell markers (CD79a, L26) in rare cases of extranodal cytotoxic peripheral T- (NK/T-) cell lymphomas. Mod. Pathol. 2000;13(7):766-772. doi:10.1038/modpathol.3880133
- Barry TS, Jaffe ES, Sorbara L, Raffeld M, Pittaluga S. Peripheral T-cell lymphomas expressing CD30 and CD15. Am J Surg Pathol. 2003;27(12):1513-1522. doi:10.1097/00000478-200312000-00003
- Bailey NG, Elenitoba-Johnson KSJ. Molecular diagnostics of T-cell lymphoproliferative disorders. Cancer J. 2014;20(1):48-60. doi:10.1097/PPO.0000000000000016
- Groenen PJTA, Langerak AW, van Dongen JJM, van Krieken JHJM. Pitfalls in TCR gene clonality testing: teaching cases. J Hematop. 2008;1(2):97-109. doi:10.1007/s12308-008-0013-9
- Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251-264. doi:10.1016/j.jmoldx.2014.12.006
- Couronné L, Bastard C, Bernard OA. TET2 and DNMT3A mutations in human T-cell lymphoma. N Engl J Med. 2012;366(1):95-96. doi:10.1056/NEJMc1111708
- Lemonnier F, Couronné L, Parrens M, et al. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood. 2012;120(7):1466-1469. doi:10.1182/blood-2012-02-408542
- Cairns RA, Iqbal J, Lemonnier F, et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood. 2012;119(8):1901-1903. doi:10.1182/blood-2011-11-391748
- Odejide O, Weigert O, Lane AA, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123(9):1293-1296. doi:10.1182/blood-2013-10-531509
- Wang C, McKeithan TW, Gong Q, et al. IDH2R172 mutations define a unique subgroup of patients with angioimmunoblastic T-cell lymphoma. Blood. 2015;126(15):1741-1752. doi:10.1182/blood-2015-05-644591
- Sakata-Yanagimoto M, Enami T, Yoshida K, et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46(2):171-175. doi:10.1038/ng.2872
- Palomero T, Couronné L, Khiabanian H, et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat Genet. 2014;46(2):166-170. doi:10.1038/ng.2873
- Pedersen MB, Hamilton-Dutoit SJ, Bendix K, et al. DUSP22 and TP63 rearrangements predict outcome of ALK-negative anaplastic large cell lymphoma: a Danish cohort study. Blood. 2017;130(4):554-557. doi:10.1182/blood-2016-12-755496
- Streubel B, Vinatzer U, Willheim M, Raderer M, Chott A. Novel t(5;9)(q33;q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma. Leukemia. 2006;20(2):313-318. doi:10.1038/sj.leu.2404045
- Huang Y, Moreau A, Dupuis J, et al. Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH) and may show overlapping features with angioimmunoblastic T-cell lymphomas. Am J Surg Pathol. 2009;33(5):682-690. doi:10.1097/PAS.0b013e3181971591
- Johnson WT, Ganesan N, Epstein-Peterson ZD, et al. TP53 mutations identify high-risk peripheral T-cell lymphoma patients treated with CHOP-based chemotherapy. Blood. 2021;138(suppl 1):1367. doi:10.1182/blood-2021-151779
- Rodríguez M, Alonso-Alonso R, Tomás-Roca L, et al. Peripheral T-cell lymphoma: molecular profiling recognizes subclasses and identifies prognostic markers. Blood Adv. 2021;5(24):5588-5598. doi:10.1182/bloodadvances.2021005171
- Iqbal J, Wright G, Wang C, et al; Lymphoma Leukemia Molecular Profiling Project and the International Peripheral T-cell Lymphoma Project. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014;123(19):2915-2923. doi:10.1182/blood-2013-11-536359
- Schützinger C, Esterbauer H, Hron G, et al. Prognostic value of T-cell receptor gamma rearrangement in peripheral blood or bone marrow of patients with peripheral T-cell lymphomas. Leuk Lymphoma. 2008;49(2):237-246. doi:10.1080/10428190701784409
- Gallamini A, Stelitano C, Calvi R, et al; Intergruppo Italiano Linfomi. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103(7):2474-2479. doi:10.1182/blood-2003-09-3080
- Cheson BD, Fisher RI, Barrington SF, et al; Alliance; Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphhoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin's Study Group; Japanese Lymphorra Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. doi:10.1200/JCO.2013.54.8800
- Feeney J, Horwitz S, Gönen M, Schöder H. Characterization of T-cell lymphomas by FDG PET/CT. AJR Am J Roentgenol. 2010;195(2):333-340. doi:10.2214/AJR.09.3665
- Strouse C, Rutherford SC, Smith BJ, et al. Utility and patterns of use of PET/CT and bone marrow biopsy for staging in non-Hodgkin lymphoma in the clinical setting: a retrospective analysis using the LEO database. Blood. 2019;134(suppl 1):1610. doi:10.1182/blood-2019-126068
- Koh Y, Lee JM, Woo G-U, et al. FDG PET for evaluation of bone marrow status in T-cell lymphoma. Clin Nucl Med. 2019;44(1):4-10. doi:10.1097/RLU.0000000000002320
- Gutiérrez-García G, García-Herrera A, Cardesa T, et al. Comparison of four prognostic scores in peripheral T-cell lymphoma. Ann Oncol. 2011;22(2):397-404. doi:10.1093/annonc/mdq359
- Gleeson M, Peckitt C, Mong To Y, et al. CHOP versus GEM-P in previously untreated patients with peripheral T-cell lymphoma (CHEMO-T): a phase 2, multicentre, randomised, open-label trial. Lancet Haematol. 2018;5(5):e190-e200. doi:10.1016/S2352-3026(18)30039-5
- Mahadevan D, Unger JM, Spier CM, et al. Phase 2 trial of combined cisplatin, etoposide, gemcitabine, and methylprednisolone (PEGS) in peripheral T-cell non-Hodgkin lymphoma: Southwest Oncology Group Study S0350. Cancer. 2013;119(2):371-379. doi:10.1002/cncr.27733
- Li L, Duan W, Zhang L, et al. The efficacy and safety of gemcitabine, cisplatin, prednisone, thalidomide versus CHOP in patients with newly diagnosed peripheral T-cell lymphoma with analysis of biomarkers. Br J Haematol. 2017;178(5):772-780. doi:10.1111/bjh.14763
- Simon A, Peoch M, Casassus P, et al. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. results of the randomized phase III trial GOELAMS-LTP95. Br J Haematol. 2010;151(2):159-166. doi:10.1111/j.1365-2141.2010.08329.x
- Horwitz S, O’Connor OA, Pro B, et al; ECHELON-2 Study Group. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229-240. doi:10.1016/S0140-6736(18)32984-2
- Bachy E, Camus V, Thieblemont C, et al. Romidepsin plus CHOP versus CHOP in patients with previously untreated peripheral T-cell lymphoma: results of the Ro-CHOP phase III study (conducted by LYSA). J Clin Oncol. 2022;40(3):242-251. doi:10.1200/JCO.21.01815
- Pfreundschuh M, Trümper L, Kloess M, et al; German High-Grade Non-Hodgkin’s Lymphoma Study Group. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: results of the NHL-B1 trial of the DSHNHL. Blood. 2004;104(3):626-633. doi:10.1182/blood-2003-06-2094
- Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418-3425. doi:10.1182/blood-2010-02-270785
- Maeda Y, Nishimori H, Yoshida I, et al. Dose-adjusted EPOCH chemotherapy for untreated peripheral T-cell lymphomas: a multicenter phase II trial of West-JHOG PTCL0707. Haematologica. 2017;102(12):2097-2103. doi:10.3324/haematol.2017.167742
- Herrera AF, Zain J, Savage KJ, et al. Brentuximab Vedotin Plus Cyclophosphamide, Doxorubicin, Etoposide, and Prednisone (CHEP-BV) Followed By BV Consolidation in Patients with CD30-Expressing Peripheral T-Cell Lymphomas. Blood. 2021;138(Supplement 1):133. doi:10.1182/blood-2021-151105
- Bossard C, Dobay MP, Parrens M, et al. Immunohistochemistry as a valuable tool to assess CD30 expression in peripheral T-cell lymphomas: high correlation with mRNA levels. Blood. 2014;124(19):2983-2986. doi:10.1182/blood-2014-07-584953
- Pro B, Advani R, Brice P, et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2017;130(25):2709-2717. doi:10.1182/blood-2017-05-780049
- Fanale MA, Horwitz SM, Forero-Torres A, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32(28):3137-3143. doi:10.1200/JCO.2013.54.2456
- Horwitz SM, O’Connor OA, Pro B, et al. The Echelon-2 trial: 5-year results of a randomized,double-blind, phase 3 study of brentuximab vedotin and CHP (A+CHP) versus CHOP in frontline treatment of patients with CD30-positive peripheral T-cell lymphoma. Blood. 2020;136(suppl 1):3-5. doi:10.1182/blood-2020-134398
- Jagadeesh D, Horwitz SM, Bartlett NL, et al. Response to brentuximab vedotin by CD30 expression: results from five trials in PTCL, CTCL, and B-cell lymphomas. J Clin Oncol. 2019;37(suppl 15):7543. doi:10.1200/JCO.2019.37.15_suppl.7543
- Savage KJ, Horwitz SM, Advani RH, et al. An exploratory analysis of brentuximab vedotin plus CHP (A+CHP) in the frontline treatment of patients with CD30+ peripheral T-cell lymphomas (ECHELON-2): impact of consolidative stem cell transplant. Blood. 2019;134(supplement 1):464. doi:10.1182/blood-2019-122781
- Corradini P, Tarella C, Zallio F, et al. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia. 2006;20(9):1533-1538. doi:10.1038/sj.leu.2404306
- Rodríguez J, Conde E, Gutiérrez A, et al; Grupo Español de Linfomas/Trasplante Autólogo de Médula Osea (GEL-TAMO). Frontline autologous stem cell transplantation in high-risk peripheral T-cell lymphoma: a prospective study from the GEL-TAMO Study Group. Eur J Haematol. 2007;79(1):32-38. doi:10.1111/j.1600-0609.2007.00856.x
- Mercadal S, Briones J, Xicoy B, et al; Grup per l’Estudi dels Limfomes de Catalunya I Balears (GELCAB). Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol. 2008;19(5):958-963. doi:10.1093/annonc/mdn022
- d’Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093-3099. doi:10.1200/JCO.2011.40.2719
- Wilhelm M, Smetak M, Reimer P, et al. First-line therapy of peripheral T-cell lymphoma: extension and long-term follow-up of a study investigating the role of autologous stem cell transplantation. Blood Cancer J. 2016;6(7):e452. doi:10.1038/bcj.2016.63
- Park SI, Horwitz SM, Foss FM, et al; COMPLETE Investigators. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: report from COMPLETE, a prospective, multicenter cohort study. Cancer. 2019;125(9):1507-1517. doi:10.1002/cncr.31861
- D’Amore F, Relander T, Lauritzsen G, et al. Ten years median follow-up of the NORDIC NLG-T-01 trial on CHOEP and upfront autologous transplantation in peripheral T-cell lymphomas. Hematol Oncol. 2015;33(suppl S1):074.
- Fossard G, Broussais F, Coelho I, et al. Role of up-front autologous stem-cell transplantation in peripheral T-cell lymphoma for patients in response after induction: an analysis of patients from LYSA centers. Ann Oncol. 2018;29(3):715-723. doi:10.1093/annonc/mdx787
- Feyler S, Prince HM, Pearce R, et al. The role of high-dose therapy and stem cell rescue in the management of T-cell malignant lymphomas: a BSBMT and ABMTRR study. Bone Marrow Transplant. 2007;40(5):443-450. doi:10.1038/sj.bmt.1705752.
- Smith SM, Burns LJ, van Besien K, et al. Hematopoietic cell transplantation for systemic mature T-cell non-Hodgkin lymphoma. J Clin Oncol. 2013;31(25):3100-3109. doi:10.1200/JCO.2012.46.0188
- Mehta N, Maragulia JC, Moskowitz A, et al. A retrospective analysis of peripheral T-cell lymphoma treated with the intention to transplant in the first remission. Clin Lymphoma Myeloma Leuk. 2013;13(6):664-670. doi:10.1016/j.clml.2013.07.005
- Ellin F, Landström J, Landström L, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014;124(10):1570-1577. doi:10.1182/blood-2014-04-573089
- Parrilla Castellar ER, Jaffe ES, Said JW, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014;124(9):1473-1480. doi:10.1182/blood-2014-04-571091
- Feldman AL, Dogan A, Smith DI, et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively parallel genomic sequencing. Blood. 2011;117(3):915-919. doi:10.1182/blood-2010-08-303305
- Pedersen MB, Relander T, Fossum Lauritzsen G, et al. The impact of upfront autologous transplant on the survival of adult patients with ALCL and PTCL-NOS according to their ALK , DUSP22 and TP63 gene rearrangement status - a joined Nordic Lymphoma Group and Mayo Clinic analysis. Blood. 2017;130(suppl 1):822. doi:10.1182/blood.V130.Suppl_1.822.822
- Hapgood G, Ben‐Neriah S, Mottok A, et al. Identification of high‐risk DUSP22‐rearranged ALK ‐negative anaplastic large cell lymphoma. Br J Haematol. 2019;186(3):e28-e31. doi:10.1111/bjh.15860
- Schmitz N, Truemper LH, Bouabdallah K, et al. A randomized phase 3 trial of autologous vs. allogeneic transplantation as part of first-line therapy in poor-risk peripheral T-NHL. Blood. 2020;137(19):2646-2656. doi:10.1182/blood.2020008825
- Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048-3058. doi:10.1200/JCO.2013.53.5229
- Mehta-Shah N, Ito K, Bantilan K, et al. Baseline and interim functional imaging with PET effectively risk stratifies patients with peripheral T-cell lymphoma. Blood Adv. 2019;3(2):187-197. doi:10.1182/bloodadvances.2018024075
- Mehta-Shah N, Fehniger TA, Jacobsen ED, et al. End of treatment peripheral blood T-cell receptor gene rearrangement evaluation for minimal residual disease evaluation in peripheral T-cell lymphomas. Blood. 2020;136(Suppl 1):30-31. doi:10.1182/blood-2020-138768
- Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31(16):1970-1976. doi:10.1200/JCO.2012.44.7524
- O’Connor OA, Horwitz S, Masszi T, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol. 2015;33(23):2492-2499. doi:10.1200/JCO.2014.59.2782
- Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190-2196. doi:10.1200/JCO.2011.38.0402
- Horwitz SM, Advani RH, Bartlett NL, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123(20):3095-3100. doi:10.1182/blood-2013-12-542142
- Shi Y, Dong M, Hong X, et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26(8):1766-1771. doi:10.1093/annonc/mdv237
- Maruyama D, Tsukasaki K, Uchida T, et al. Multicenter phase 1/2 study of forodesine in patients with relapsed peripheral T cell lymphoma. Ann Hematol. 2019;98(1):131-142. doi:10.1007/s00277-018-3418-2
- O’Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29(9):1182-1189. doi:10.1200/JCO.2010.29.9024
- Piekarz RL, Frye R, Prince HM, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117(22):5827-5834. doi:10.1182/blood-2010-10-312603
- Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30(6):631-636. doi:10.1200/JCO.2011.37.4223
- Ghione P, Faruque P, Mehta-Shah N, et al. T follicular helper phenotype predicts response to histone deacetylase inhibitors in relapsed/refractory peripheral T-cell lymphoma. Blood Adv. 2020;4(19):4640-4647. doi:10.1182/bloodadvances.2020002396
- Shustov AR, Shinohara MM, Dakhil SR, Bhat G, Zain JM. Management of mucositis with the use of leucovorin as adjunct to pralatrexate in treatment of peripheral T-cell lymphomas (PTCL) — results from a prospective multicenter phase 2 clinical trial. Blood. 2018;132(suppl 1):2910. doi:10.1182/blood-2018-99-119684.
- Horwitz S, Moskowitz C, Kewalramani T, et al. Second-line therapy with ICE followed by high dose therapy and autologous stem cell transplantation for relapsed/refractory peripheral T-cell lymphomas: minimal benefit when analyzed by intent to treat. Blood. 2005;106(11):2679. doi:10.1182/blood.V106.11.2679.2679
- Rigacci L, Fabbri A, Puccini B, et al. Oxaliplatin-based chemotherapy (dexamethasone, high-dose cytarabine, and oxaliplatin)±rituximab is an effective salvage regimen in patients with relapsed or refractory lymphoma. Cancer. 2010;116(19):4573-4579. doi:10.1002/cncr.25216
- Park B-B, Kim WS, Suh C, et al. Salvage chemotherapy of gemcitabine, dexamethasone, and cisplatin (GDP) for patients with relapsed or refractory peripheral T-cell lymphomas: a Consortium for Improving Survival of Lymphoma (CISL) trial. Ann Hematol. 2015;94(11):1845-1851. doi:10.1007/s00277-015-2468-y
- Enblad G, Hagberg H, Erlanson M, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004;103(8):2920-2924. doi:10.1182/blood-2003-10-3389
- Damaj G, Gressin R, Bouabdallah K, et al. Results from a prospective, open-label, phase II trial of bendamustine in refractory or relapsed T-cell lymphomas: the BENTLY trial. J Clin Oncol. 2013;31(1):104-110. doi:10.1200/JCO.2012.43.7285
- Zinzani PL, Musuraca G, Tani M, et al. Phase II trial of proteasome inhibitor bortezomib in patients with relapsed or refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25(27):4293-4297. doi:10.1200/JCO.2007.11.4207
- Advani R, Horwitz S, Zelenetz A, Horning SJ. Angioimmunoblastic T cell lymphoma: treatment experience with cyclosporine. Leuk Lymphoma. 2007;48(3):521-525. doi:10.1080/10428190601137658
- Zinzani PL, Magagnoli M, Bendandi M, et al. Therapy with gemcitabine in pretreated peripheral T-cell lymphoma patients. Ann Oncol. 1998;9(12):1351-1353. doi:10.1023/a:1008409601731
- Toumishey E, Prasad A, Dueck G, et al. Final report of a phase 2 clinical trial of lenalidomide monotherapy for patients with T-cell lymphoma. Cancer. 2015;121(5):716-723. doi:10.1002/cncr.29103
- Stuver RN, Khan N, Schwartz M, et al. Single agents vs combination chemotherapy in relapsed and refractory peripheral T-cell lymphoma: results from the Comprehensive Oncology Measures for Peripheral T-cell Lymphoma Treatment (COMPLETE) registry. Am J Hematol. 2019;94(6):641-649. doi:10.1002/ajh.25463
- Ma H, Cheng B, Falchi L, et al. Survival benefit in patients with peripheral T-cell lymphomas after treatments with novel therapies and clinical trials. Hematol Oncol. 2020;38(1):51-58. doi:10.1002/hon.2705
- Ghosh N, Ahmed S, Ahn KW, et al. Association of reduced-intensity conditioning regimens with overall survival among patients with non-Hodgkin lymphoma undergoing allogeneic transplant. JAMA Oncol. 2020;6(7):1011-1018. doi:10.1001/jamaoncol.2020.1278
- Mehta-Shah N, Kommalapati A, Teja S, et al. Successful treatment of mature T-cell lymphoma with allogeneic stem cell transplantation: the largest multicenter retrospective analysis. Blood. 2020;136(suppl 1):35-36. doi:10.1182/blood-2020-138542
- Bellei M, Foss FM, Shustov AR, et al; International T-cell Project Network. The outcome of peripheral T-cell lymphoma patients failing first-line therapy: a report from the prospective, International T-cell Project. Haematologica. 2018;103(7):1191. doi:10.3324/haematol.2017.186577
- Du J, Yu D, Han X, Zhu L, Huang Z. Comparison of allogeneic stem cell transplant and autologous stem cell transplant in refractory or relapsed peripheral T-cell lymphoma: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(5):e219807. doi:10.1001/jamanetworkopen.2021.9807
- Ghosh N, Karmali R, Rocha V, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors versus HLA-matched sibling donors: a Center for International Blood and Marrow Transplant Research analysis. J Clin Oncol. 2016;34(26):3141-3149. doi:10.1200/JCO.2015.66.3476
- Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127(7):938-947. doi:10.1182/blood-2015-09-671834
- Hamadani M, Ngoya M, Sureda A, et al. Outcome of allogeneic transplantation for mature T-cell lymphomas: impact of donor source and disease characteristics. Blood Adv. 2022;6(3):920-930. doi:10.1182/bloodadvances.2021005899
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49(11):1360-1365. doi:10.1038/bmt.2014.161
- Hosing C, Bassett R, Dabaja B, et al. Allogeneic stem-cell transplantation in patients with cutaneous lymphoma: updated results from a single institution. Ann Oncol. 2015;26(12):2490-2495. doi:10.1093/annonc/mdv473
- Weng W-K, Arai S, Rezvani A, et al. Nonmyeloablative allogeneic transplantation achieves clinical and molecular remission in cutaneous T-cell lymphoma. Blood Adv. 2020;4(18):4474-4482. doi:10.1182/bloodadvances.2020001627
- Domingo-Domenech E, Duarte RF, Boumedil A, et al. Allogeneic hematopoietic stem cell transplantation for advanced mycosis fungoides and Sézary syndrome. an updated experience of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2021;56(6):1391-1401. doi:10.1038/s41409-020-01197-3
- Mori T, Shiratori S, Suzumiya J, et al. Outcome of allogeneic hematopoietic stem cell transplantation for mycosis fungoides and Sézary syndrome. Hematol Oncol. 2020;38(3):266-271. doi:10.1002/hon.2719
- Delioukina M, Zain J, Palmer JM, Tsai N, Thomas S, Forman S. Reduced-intensity allogeneic hematopoietic cell transplantation using fludarabine-melphalan conditioning for treatment of mature T-cell lymphomas. Bone Marrow Transplant. 2012;47(1):65-72. doi:10.1038/bmt.2011.16
- Czajczynska A, Günther A, Repp R, et al. Allogeneic stem cell transplantation with BEAM and alemtuzumab conditioning immediately after remission induction has curative potential in advanced T-cell non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2013;19(11):1632-1637. doi:10.1016/j.bbmt.2013.07.003
- Kyriakou C, Canals C, Finke J, et al. Allogeneic stem cell transplantation is able to induce long-term remissions in angioimmunoblastic T-cell lymphoma: a retrospective study from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2009;27(24):3951-3958. doi:10.1200/JCO.2008.20.4628
- Jacobsen ED, Kim HT, Ho VT, et al. A large single-center experience with allogeneic stem-cell transplantation for peripheral T-cell non-Hodgkin lymphoma and advanced mycosis fungoides/Sezary syndrome. Ann Oncol. 2011;22(7):1608-1613. doi:10.1093/annonc/mdq698
- Dodero A, Spina F, Narni F, et al. Allogeneic transplantation following a reduced-intensity conditioning regimen in relapsed/refractory peripheral T-cell lymphomas: long-term remissions and response to donor lymphocyte infusions support the role of a graft-versus-lymphoma effect. Leukemia. 2012;26(3):520-526. doi:10.1038/leu.2011.240
- Le Gouill S, Milpied N, Buzyn A, et al; Société Francaise de Greffe de Moëlle et de Thérapie Cellulaire.. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Société Francaise de Greffe de Moëlle et de Thérapie Cellulaire. J Clin Oncol. 2008;26(14):2264-2271. doi:10.1200/JCO.2007.14.1366
- Zain J, Palmer JM, Delioukina M, et al. Allogeneic hematopoietic cell transplant for peripheral T-cell non-Hodgkin lymphoma results in long-term disease control. Leuk Lymphoma. 2011;52(8):1463-1473. doi:10.3109/10428194.2011.574754
- Goldberg JD, Chou JF, Horwitz SM, et al. Long-term survival in patients with peripheral T-cell non-Hodgkin lymphomas after allogeneic hematopoietic stem cell transplant. Leuk Lymphoma. 2012;53(6):1124–1129. doi:10.3109/10428194.2011.645818.
- Mamez A-C, Dupont A, Blaise D, et al. Allogeneic stem cell transplantation for peripheral T cell lymphomas: a retrospective study in 285 patients from the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC). J Hematol Oncol. 2020;13(1):56. doi:10.1186/s13045-020-00892-4.
- Shustov AR, Gooley TA, Sandmaier BM, et al. Allogeneic haematopoietic cell transplantation after nonmyeloablative conditioning in patients with T-cell and natural killer-cell lymphomas. Br J Haematol. 2010;150(2):170–8. doi:10.1111/j.1365-2141.2010.08210.x
- Yuan L, Sun L, Bo J, et al. Durable remission in a patient with refractory subcutaneous panniculitis-like T-cell lymphoma relapse after allogeneic hematopoietic stem cell transplantation through withdrawal of cyclosporine. Ann Transplant. 2011;16(3):135-138. doi:10.12659/aot.882007
- Kako S, Izutsu K, Oshima K, et al. Regression of the tumor after withdrawal of cyclosporine in relapsed extranodal natural killer/T cell lymphoma following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007;82(10):937-939. doi:10.1002/ajh.20943
- Horwitz SM, Koch R, Porcu P, et al. Activity of the PI3K-δ,γ inhibitor duvelisib in a phase 1 trial and preclinical models of T-cell lymphoma. Blood. 2018;131(8):888-898. doi:10.1182/blood-2017-08-802470
- Pro B, Brammer JE, Casulo C, et al. Duvelisib in patients with relapsed/refractory peripheral T-cell lymphoma from the phase 2 PRIMO trial: dose optimization efficacy update and expansion phase initial results. Blood. 2020;136(suppl 1):38-39. doi:10.1182/blood-2020-140412
- Brammer J, Zinzani PL, Zain JM, et al. Duvelisib in patients with relapsed/refractory peripheral T-cell lymphoma from the phase 2 PRIMO trial: results of an interim analysis. Blood. 2021;138(suppl 1):2456. doi:10.1182/blood-2021-148939
- Horwitz SM, Feldman TA, Hess BT, et al. A phase 2 study of the dual SYK/JAK Inhibitor cerdulatinib demonstrates good tolerability and clinical response in relapsed/refractory peripheral T-cell lymphoma and cutaneous T-cell lymphoma. Blood. 2019;134(Suppl 1):466. doi:10.1182/blood-2019-123986
- Waldmann TA, Chen J. Disorders of the JAK/STAT pathway in T cell lymphoma pathogenesis: implications for immunotherapy. Annu Rev Immunol. 2017;35:533-550. doi:10.1146/annurev-immunol-110416-120628
- Moskowitz AJ, Ghione P, Jacobsen E, et al. A phase 2 biomarker-driven study of ruxolitinib demonstrates effectiveness of JAK/STAT targeting in T-cell lymphomas. Blood. 2021;138(26):2828-2837. doi:10.1182/blood.2021013379
- Witzig TE, Sokol L, Foss FM, et al. Proof of concept for tipifarnib in relapsed or refractory angioimmunoblastic T-cell lymphoma (AITL) and CXCL12+ peripheral T-cell lymphoma (PTCL): preliminary results from an open-label, phase 2 study. Blood. 2019;134(Suppl 1):468. doi:10.1182/blood-2019-128513
- Morishima S, Ishitsuka K, Izutsu K, et al. First-in-human study of the EZH1/2 dual inhibitor valemetostat in relapsed or refractory non-Hodgkin lymphoma (NHL) - updated results focusing on adult T-cell leukemia-lymphoma (ATL). Blood. 2019;134(Suppl 1):4025. doi:10.1182/blood-2019-125507
- Yoshimitsu M, Izutsu K, Makita S, et al. Pivotal phase 2 study of the EZH1 and EZH2 Inhibitor valemetostat tosylate (DS-3201b) in patients with relapsed or refractory adult T-cell leukemia/lymphoma. Blood. 2021;138(Suppl 1):303. doi:10.1182/blood-2021-146657
- FDA approves crizotinib for childrena and young adults with relapsed or refractory, systemic anaplastic large cell lymphoma. FDA. January 14, 2021. Accessed April 5, 2022. https://bit.ly/3uYOsS2
- Fukano R, Mori T, Sekimizu M, et al. Alectinib for relapsed or refractory anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: an open-label phase II trial. Cancer Sci. 2020;111(12):4540-4547. doi:10.1111/cas.14671
- Horwitz SM, Nikitina A, Kotlov N, et al. The combination of duvelisib and romidepsin (DR) is highly active against relapsed/refractory peripheral T-cell lymphoma with low rates of transaminitis: final results and biomarker analysis. Blood. 2021;138(Suppl 1):3847. doi:10.1002/hon.56_2879
- Mehta-Shah N, Lunning MA, Moskowitz AJ, et al. Romidepsin and lenalidomide-based regimens have efficacy in relapsed/refractory lymphoma: combined analysis of two phase I studies with expansion cohorts. Am J Hematol. 2021;96(10):1211-1222. doi:10.1002/ajh.26288
- Ahmed S, Flinn IW, Mei M, et al. Safety and efficacy profile of autologous CD30.CAR-T-cell therapy in patients with relapsed or refractory classical hodgkin lymphoma (CHARIOT trial). Blood. 2021;138(suppl 1):3847. doi:10.1182/blood-2021-146100
- Ramos CA, Ballard B, Zhang H, et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J Clin Invest. 2017;127(9):3462-3471. doi:10.1172/JCI94306
- Grover NS, Ivanova A, Moore DT, et al. CD30-directed CAR-T cells co-expressing CCR4 in relapsed/refractory hodgkin lymphoma and CD30+ cutaneous T Cell lymphoma. Blood. 2021;138(suppl 1):742. doi:10.1182/blood-2021-148102
- Rouce RH, Hill LC, Smith TS, et al. Early signals of anti-tumor efficacy and safety with autologous CD5.CAR T-cells in patients with refractory/relapsed T-cell lymphoma. Blood. 2021;138(suppl 1):654. doi:10.1182/blood-2021-154142

Highlighting Insights From the Marginal Zone Lymphoma Workshop
Clinicians outline the significance of the MZL Workshop, where a gathering of international experts in the field discussed updates in the disease state.