Expert Commentary On The Product Profile Of Dostarlimab
In an interview with ONCOLOGY®, Megan May, PharmD, BCOP, offers a comprehensive review of real-world treatment considerations of dostarlimab as therapy for adult patients with recurrent or advanced solid tumors with deficient mismatch repair.
Megan May, PharmD, BCOP
Clinical Oncology Pharmacy Specialist Baptist Health
Lexington, KY

ONCOLOGY®:Can you describe the mechanism of action of dostarlimab?
May: Dostarlimab is a PD-1 antibody. It is a humanized monoclonal antibody of the IgG4 isotype that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2. This causes a release in the PD-1 pathway through mediated inhibition of the immune response.
Q: What are some of the biggest concerns with dostarlimab’s toxicity profile and have any become more apparent in the real-world setting?
May: In the real world, dostarlimab has been well tolerated. However, there are some significant immune-related adverse effects that do occur. We need to keep these in mind. When we’re counseling patients prior to the start of a therapy, we need the patients to be able to identify these immune-related adverse effects early so we’re able to quickly manage them. These immune-mediated reactions can occur in any organ system or tissue. Most of the
immune-mediated reactions occurred in less than 5% of the patients and the most common ones were fatigue, nausea, and anemia. It’s also important that we monitor the patient’s bloodwork, including their liver, renal, and thyroid function. We also need to [inform] the patient that they need to let their health care team know if they did develop any symptoms, such as a cough or shortness of breath, which could be a sign of pneumonitis, and to let us know if they develop diarrhea, which could be a sign of colitis.
Q: Are dosing modifications common with this agent?
May: [There are no] dose reductions for dostarlimab that are recommended.
Either you give the drug or you don’t give it. In general, you withhold dostarlimab for severe or grade 3 immune-mediated adverse reactions.
Q: Should clinicians be aware of any drug interactions?
May: It is recommended to monitor therapy with dostarlimab if a patient is also taking an antibiotic or a proton pump inhibitor, [as] both of these can reduce the therapeutic effect of dostarlimab. Ketoconazole can also enhance the hepatic toxicity effect of dostarlimab, so…monitoring for liver function [is recommended]. Then, just [as with] all immune checkpoint inhibitors, you should evaluate the need of a steroid in patients.
Q: Have there been any barriers to administration or receipt by the patient since the approval, such as common reimbursement issues or logistical challenges?
May: In my clinic, I have not had any issues with reimbursement for dostarlimab. It was originally approved for the treatment of mismatch repair deficient [dMMR] recurrent or advanced endometrial cancer in patients who have progressed on or [had] prior treatments with a platinum-containing regimen.3 The more recent approval is for the treatment of dMMR recurrent or advanced solid tumors.4 This most recent approval has [expanded] the indication to any solid tumor with dMMR. If a patient has this mutation, then reimbursement really should not be an issue. In relation to the logistics with dostarlimab, [the drug] is infused over 30 minutes through a 0.2-µm low-protein–binding in-line or add-on filter, and it should be given with PVC intravenous tubing. It should be stored in the original container until the time of preparation to protect it from light.
Q: Is there anything else you want to discuss?
May: A unique part of this medication is its dosing regimen. The first 4 treatments are administered every 3 weeks, and then the treatments are administered every 6 weeks. This dosing regimen was based on the receptor occupancy and the pharmacokinetic data that showed that this dosing regimen provides sufficient serum concentration to achieve and maintain the maximal receptor occupancy throughout both intervals. This unique dosing schedule allows for less frequent clinic visits after 12 weeks of initial treatment with dostarlimab.
It’s important to know that dostarlimab was approved based on an accelerated approval. The confirmatory study is currently [underway]. [The drug] has also been evaluated in the frontline setting in combination with chemotherapy, and in a large, randomized placebo-controlled phase 3 study called the RUBY trial [NCT03981796]. These data will show us if we can change the frontline treatment algorithm of endometrial cancer even further.
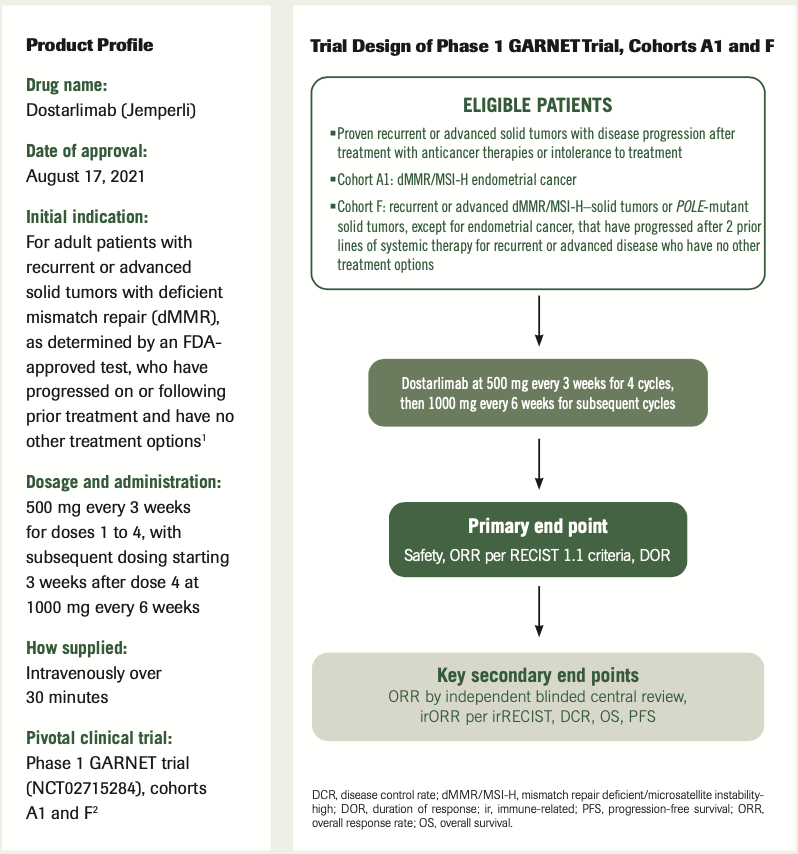
Trial Design of Phase 1 GARNET Trial, Cohorts A1 and F
References
- Jemperli. Prescribing information. GlaxoSmithKline; 2021. Accessed April 12, 2022. https://bit.ly/3Kwfkzs
- GSK receives FDA accelerated approval for JEMPERLI (dostarlimab-gxly) for adult patients with mismatch repair-deficient (dMMR) recurrent or advanced solid tumours. News release. GlaxoSmithKline. August 17, 2021. Accessed April 13, 2022. https://bit.ly/3jAHFJi
- FDA grants accelerated approval to dostarlimab-gxly for dMMR endometrial cancer. News release. FDA. April 22, 2021. Accessed April 12, 2022. https://bit.ly/376ya1S
- FDA grants accelerated approval to dostarlimab-gxly for dMMR advanced solid tumors. News release. FDA. August 17, 2021. Accessed April 12, 2022. https://bit.ly/3JERKz7

How Supportive Care Methods Can Improve Oncology Outcomes
Experts discussed supportive care and why it should be integrated into standard oncology care.