Cancer-Related Fatigue Outcome Measures in Integrative Oncology: Evidence for Practice and Research Recommendations
This review article written by Danielle Gentile, PhD, et al, reviews the management of cancer-related fatigue in integrative oncology.
ABSTRACT
Cancer-related fatigue (CRF) is one of the most common symptoms across the cancer continuum and is often underreported and undertreated. Defined as a distressing, persistent, subjective sense of tiredness or exhaustion related to cancer or its treatment, CRF includes physical, emotional, cognitive, and spiritual dimensions. Patient-reported outcome (PRO) measures are the most widely used tool to screen for and assess fatigue and the associated negative impacts on quality of life. However, selecting subjective CRF measures can be complex. This has resulted in the availability of and inconsistent use of numerous PROs, limiting the ability to cross-compare outcomes clinically and within research. To address this, the PROs that are most widely reported in the literature are recommended to support the standardization of a core set of validated measures. The National Comprehensive Cancer Network single-item tool for clinical significance is recommended for quick use in clinical environments; the Brief Fatigue Inventory allows for fast, easy, helpful cutoffs on severity threshold for triage, and measures both severity and interference with daily functioning; while the MD Anderson Symptom Inventory allows for multisymptomatic assessment. In addition, a fundamental consideration for any PRO use is the administrative burden on the patient and clinician. In this review, we aim to summarize current, validated PROs specific to CRF to aid clinicians and researchers in patient care and in study design and implementation. We conclude with suggestions for future directions in CRF research that can increase the possibility for long-term impact on future guidelines of fatigue management.
Oncology (Williston Park). 2022;36(5):276-287.
DOI: 10.46883/2022.25920958
Introduction
The National Comprehensive Cancer Network (NCCN) guidelines1 define cancer-related fatigue (CRF) as “a distressing, persistent, subjective sense of tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.” For patients undergoing treatment, different chemotherapy agents, surgery, and radiation can cause acute or persistent CRF that impacts how tolerable they find their therapy. CRF can be either short- or long-term, with as many as one-fourth to one-third of survivors experiencing CRF for up to 10 years after cancer treatment.2 It is widely understood to be a multidimensional symptom assessed by clinicians and researchers for presence, severity, impact on quality of life (QOL), and interference in activities of daily living. Due to the subjective and multidimensional nature of CRF, researchers should measure CRF across the emotional, cognitive, physical, and spiritual3 domains. There is great diversity in the patient-reported outcomes (PROs) used to measure CRF throughout clinical practice and research. The numerous PROs available and their inconsistent use limit the ability to cross-compare outcomes clinically and within research. In particular, the difficulty associated with the diversity of CRF measures can lead to challenges in comparing study results across populations of patients with cancer.
The Society of Integrative Oncology (SIO) is a nonprofit, multidisciplinary professional organization that promotes rigorous, evidence-based, comprehensive integrative approaches for patients with cancer and for survivors. As members of the SIO research committee, we seek to clarify CRF PROs for oncology clinicians and researchers. Therefore, in this whitepaper, we (1) define CRF and summarize CRF prevalence, impacts, and associations across the cancer continuum; (2) discuss the challenges for diagnosis and treatment, including potential mechanisms and interventions; (3) introduce CRF measurement issues; (4) summarize the CRF PRO measures most widely reported in the literature; and (5) provide recommendations to oncology clinicians and researchers on which validated CRF PRO measures to use. We will limit any discussion of objective measures to focus on recommendations for measuring patient-reported CRF. A previous whitepaper written by SIO Research Committee members on pain PROs with similar goals has been published.4
Prevalence
Cancer-related fatigue is among the most common symptoms across cancer types and is often underreported and undertreated. Patients across the cancer continuum—including those recently diagnosed or in treatment, as well as long-term survivors5—often report CRF as among their top 3 symptoms, describing it as the most distressing of all symptoms.6 CRF is present in 25% to 99% of patients who are undergoing cancer treatment2 and in 19% to 82% of patients post treatment7,8; the overall pooled prevalence is 52%.9
Studies reporting age associations with CRF have been inconsistent. For example, Al Maqbali et al10 report no association between age and CRF, whereas Alvarez-Bustos et al11 report younger people having higher levels of CRF. The latter finding is supported by a review of studies across the United States, United Kingdom, Norway, Canada, and Sweden, where 31% to 100% of adolescent and young adult (aged 13-24 years) survivors reported CRF12 as a significant and disabling problem. However, this prevalence based on age should be considered with caution in consideration of the underrepresentation of older age groups in cancer clinical trials.13,14
With respect to gender, recent meta-analyses report female patients having a higher prevalence of and risk for CRF.9,10 Prevalence can further be broken down by cancer type. An epidemiological study using multidimensional CRF measures (n = 2244) reported that patients with breast cancer had the lowest adjusted mean physical fatigue values. In contrast, those values were markedly higher in those with stomach, lung, kidney, pancreas, and endometrium cancers.2 These prevalence reports indicate variability in CRF across cancer types, and that females report CRF in higher numbers.
Negative Impacts and Associations With CRF
Those with CRF often experience reduced QOL.15 Persistent CRF limits the ability to work, complete activities of daily living, and maintain social relationships.16 Tasks such as household chores, running errands, and meeting the needs of family members can become exhausting. This results in a loss of interest in individuals’ usual activities and in reduced concentration and memory/recall.17
CRF is a comorbid symptom with other ailments including pain, sleep disturbance, anemia, cachexia, sarcopenia, mood disorders, spiritual concerns, anorexia, and various gastrointestinal symptoms (eg, mouth sores, xerostomia, abdominal pain) leading to loss of appetite.18-20 As a result, measuring CRF as an individual symptom isolated from its related conditions becomes complicated.21,22 This is particularly challenging in relation to depression as it is difficult to find fatigue and depression scales that do not contain similar concepts. For example, the Patient Health Questionnaire–9, a commonly used depression scale, has a question—“Feeling tired or having little energy”—that directly overlaps with fatigue. It also contains questions that would be expected to be impacted by either depression or fatigue, such as, “Trouble concentrating on things such as…” Across CRF dimensions, there is an inverse association between CRF and psychological resilience, which has a direct impact on improvement in QOL. This has been observed in studies23 using Antonovsky’s Sense of Coherence (SOC) scale, where resilience has a mitigating effect on the development of fatigue. SOC is directly linked to the concept of salutogenesis, which focuses on how people use coping mechanisms to maintain their health despite stressful life situations.24
Challenges for Diagnosis and Treatment
Diagnosing CRF is challenging due to the condition’s multifactorial quality, which may be further complicated by how clinicians measure and assess CRF. Researchers recommend that diagnosis be informed by 4 criteria: (1) Indicators of symptom presence should be persistent for 2 or more weeks; (2) there should be evidence of distress or impairment; (3) the distinction should be made that the condition is related to cancer or cancer treatment; and (4) there is absence of any previous or existing psychiatric disorder.21,25 This last criterion should be viewed with skepticism. This is because any preexisting or current psychiatric disorders do not rule out the development of CRF after a cancer diagnosis, in fact they can often overlap. These challenges lead to clinical guidelines calling for a differential diagnosis to rule out comorbidities, namely depression.26
While the focus of this manuscript is not CRF treatment, it is worth noting the current research in this area. No treatment mode is known to be most effective for CRF in all cases; however, a meta-analysis of randomized control trials outlines 4 major types of CRF treatments that are recommended for adult patients with cancer: exercise; psychological intervention; exercise plus psychological intervention; and pharmaceutical intervention.27 Interestingly, Mustian and colleagues27 report that certain intervention modes may be more effective for treating CRF at different points in the cancer treatment trajectory. Overall, the findings of this study demonstrate the efficacy of exercise and psychological interventions for improving CRF during and after primary treatment. With respect to pharmacological treatments, Chow and colleagues28 report findings from a systematic review and meta-analysis of 20 randomized control trials concluding that methylphenidate, modafinil, and paroxetine were superior to placebo; methylphenidate and modafinil were equivalent to one another; and paroxetine was superior to both methylphenidate and modafinil. Chow et al also stated that more safety data are required for pharmacologic interventions. A systematic review29 of randomized controlled trials on integrative therapy interventions with CRF as the primary outcome included 30 studies; the quality of trials ranged from 2 to 8, with a mean score of 5.3, with most scores between 5 and 7 (n = 20). The results indicate that cognitive behavioral therapy combined with hypnosis and American ginseng is likely to be effective in patients receiving cancer treatment. For those in the posttreatment population, acupressure, mindfulness-based interventions, and qigong/Tai Chi Easy™ were all found to be likely effective in reducing CRF. Furthermore, additional studies have demonstrated promising complementary and integrative interventions with positive results at different points across the cancer continuum, including yoga,30,31 acupuncture,32 art therapy,33,34 and mindfulness-based stress reduction.32,35 Such herbal medicines as Paullinia cupana,36-38 Uncaria tomentosa,39 Viscum album,40,41 and American and Asian ginseng,42,43 as well as some prescribed in traditional Chinese medicine,44 have evidence of safety and efficacy, although additional research with high-quality methodology is needed.
Additional insights have been gained through pragmatic research involving patients with CRF, assessing the entire integrative oncology approach. This research includes nonrandomized trials that are patient-preference controlled. Findings suggest that patient-tailored multimodality integrative oncology treatment, adapted to patients’ particular preferences and QOL-related concerns, may also alleviate CRF.45-47
Mechanisms of CRF
Because this paper focuses on the assessment of CRF, it is important to introduce the complex etiology of CRF that contributes to the challenges in its measurement. The etiology of CRF is not well understood, and this hinders progress in ways to diagnose it and in finding interventions that alleviate this persistent syndrome.48 CRF mechanisms and the associated hypotheses are divided across the central and peripheral domains. Peripheral fatigue disrupts central stimulation and muscle response; it is characterized both by a lack of adenosine triphosphate, a compound that provides energy to drive processes such as muscle contraction, and by a buildup of metabolic by-products such as lactic acid. In contrast, central fatigue extends from the central nervous system (CNS), including the brain and spinal cord. It is associated with changes in the synaptic junctions, which impact the function of neurotransmitters within the CNS. These synaptic junction changes further impact muscle function that are not explained by peripheral factors.
Central fatigue includes 5 distinct hypothesized mechanisms of action: (1) hypothalamic–pituitary–adrenal axis dysregulation, (2) circadian rhythm dysregulation, (3) serotonin dysregulation, (4) vagal afferent activities, and (5) neuroendocrine impairment.49-51 A growing interest in neuroimaging studies that focus on the disruption of brain connectivity and changes in brain metabolites may lead to a sixth hypothesis that goes beyond serotonin dysregulation.52,53 Three hypotheses about the potential mechanisms of peripheral fatigue are (1) degeneration of muscle function, (2) impaired adenosine triphosphate/contractile properties, and (3) impaired physical function.49-51 Cytokine dysregulation as a mechanism of action is hypothesized to affect both central and peripheral fatigue. To date, the most studied aspect of etiology concerns the cytokine hypothesis, demonstrating activation of proinflammatory cytokines as a common factor that leads to CRF and other behavioral changes, known as “sickness behavior.”54 One such study involved women undergoing chemotherapy for breast cancer to investigate the potential for predictors of fatigue in relation to cytokine levels.55 Earlier studies demonstrate support for other hypotheses, such as between heart rate variability and CRF56,57 and between muscle contractile properties and CRF.58
Measurement Concerns
Many factors influence the chosen CRF measurement, contributing to the lack of PRO consistency used across clinical practice and research. The varying nature of CRF is reflected in the diverse recall periods of different PROs (eg, in the last week; within the past 24 hours), varying state (eg, fatigue at its worst; on average; right now), and severity (eg, none, mild, moderate, severe). Different PRO instruments measure different constructs that reflect fatigue’s multifaceted nature, including its impact on physical function, mental focus and capacity, emotional state, and social interactions. Instruments that capture global fatigue symptoms may mask other symptoms and conditions. However, unidimensional measures59 of CRF that are subscales of more extensive, multifactorial tools can be helpful for a brief screening assessment. The measurement issues that will be discussed in greater detail are subjectivity, the intended measurement purpose, psychometric properties, the timing of assessment, methodological considerations, and differences across languages.
Subjectivity
CRF is a subjective, multifactorial experience that varies in severity and duration; it also has alleviating and aggravating factors. This pervasive symptom has predominantly been measured based on patient reports. Clinicians and researchers assess these reports for many aspects, including presence, severity, and distress, as well as distinguishing among the physical, emotional, cognitive, and spiritual dimensions of CRF.
Intended measurement purpose
The intended purpose of an instrument is also essential to consider when selecting a CRF PRO measure. Research purposes may necessitate different measures than clinical purposes. In both settings, the burden of implementation must be considered. Data collection may be burdensome for both assessor and the patient, so measures that can be completed quickly with limited disruption of the clinical workflow and an easy interpretation are often preferred. Furthermore, some measures are appropriate for brief screening, while others are more suited to support a diagnosis, intervention, or health outcome assessment of CRF. The magnitude of patient-reported symptom burden is critical when a decision must be made about which measures should be used to capture clinical meaningful symptom burden to determine CRF management. For example, the NCCN guidelines on CRF describe a 0-to-10 scale of fatigue severity and indicate that a score of 4 or greater, indicating moderate to severe fatigue severity, would call for CRF management in a clinical setting.1 The simple “Fatigue worst” severity item can be found in several validated PRO assessment tools, including the Brief Fatigue Inventory (BFI) assessment tool,60 the MD Anderson Symptom Inventory (MDASI) multiple symptom assessment tool,61 and the Edmonton Symptom Assessment System (ESAS-r), which is a widely used palliative care tool.62 Research from a multicenter study on the optimal threshold of fatigue severity category for clinically meaningful impact on functioning confirmed the NCCN guideline consensus, that a score of 4 or greater presents moderate fatigue and 7 or greater presents severe fatigue.63 The clinical actionability of measurement results can often be unclear. The availability and cost of measures are an additional concern. Some are available for no-cost public use, while others require payment for licensing fees. Researchers should consider these crucial aspects when making decisions about which PRO to use in a study.
Psychometric properties
The available psychometric properties of reliability, validity, and sensitivity are important for making PRO use decisions. Establishing the validity of an instrument is an ongoing process, and studies assessing validity are integral to clarify the purposes and contexts in which PROs are suitable. It is also vital to understand that an instrument may not be valid across different settings or contexts. Examples of these purposes or contexts include predicting treatment outcomes in a particular type of cancer or treatment phase, discriminating between fatigue and depression, or using measures in specific populations. Where possible, it is advisable to compare findings of different studies with similar contexts and populations to rule out any concern of a PRO being used due to popularity over performance.64
Timing of assessment
Another measurement issue concerns the question of the timing of fatigue assessment, especially during the trajectory of disease or treatment directly impacting CRF severity. It is necessary to differentiate among clinical settings: sometimes, for instance, fatigue is relatively “stable” (eg, during survivorship), and at other times, fatigue “spikes” as a result of oncology treatment. This can happen following stem cell transplant, major surgery, or a chemotherapy protocol, such as when fatigue emerges in “pulses” that rise at chemo days 2 to 3 and improve toward days 7 to 10. In these cases, chemotherapy-related fatigue may drive cancer-related fatigue. When subjects are undergoing chemotherapy, the timing of the baseline and follow-up CRF and health-related QOL (HRQOL) assessments is critical. In those cases where chemotherapy toxicity is the main fatigue etiology, there is a need to establish “optimality of assessment”45 to ensure that clinicians keep a similar time interval between the administration of chemotherapy (baseline) and the follow-up assessments. So, if an initial assessment was performed at chemotherapy day 3, the next assessment(s) should also be timed to day 3 of the next chemotherapy cycle(s). Provided that the chemotherapy regimen remains unchanged, clinicians could consider the assessment optimal if it took place within a similar interval between the administration of chemotherapy and the follow-up visit at baseline and the follow-up assessments.45 Clinicians and researchers should carefully consider measurements that align with the timing of key aspects of the patient’s experience. For example, it may be necessary to measure the development of CRF at cancer diagnosis before treatment and then throughout treatment (eg, weekly) or measure CRF after an intervention has been initiated to assess its efficacy. The latter assessment would need to be tailored depending on the therapy initiated.
Methodological considerations
Finally, Wang points out the methodological concerns associated with “response shift.”51 This concept concerns the point at which patients experience fatigue such that it changes how they judge the severity or intensity of CRF. This response shift can lead to underreporting of CRF, because patients may adapt their internal standards, values, or lifestyles to their illness perception. As a result, tracking changes in CRF over time can represent a response shift rather than a reduction of the symptom. Alternatively, it could represent a response (or lack thereof) to treatment. To differentiate between response shift and treatment response and address the potential for negating internal validity, Howard and colleagues65 recommend extending the traditional pretest-posttest study design with a “thentest.” In this scenario, respondents are asked to recall the point in time at which the pretest was administered, thereby giving a renewed judgment on their pretest levels. This method has been used to assess response shift in patients undergoing radiation for cancer who report CRF.66
Language challenges
Before the NCCN guidelines for fatigue were established, research has demonstrated that patient language is a challenge for CRF measurement. Not everyone uses similar words to describe their CRF experiences,67 and this creates further problems in diagnosing CRF’s presence or severity. In other words, the distinctions that different people make among exhaustion, tiredness, weakness, fatigue, and lack of energy is unclear.49 A study that sought to identify clinical associations of 3 fatigue word descriptors—“fatigue,” “weakness,” and “lack of energy”—found that these terms are not synonymous.68 For instance, studies of PRO measures have found that fatigue is only inherently understood in English and French6 and key fatigue terms (weakness, tired, energy) are understood and used differently across cultures, generations, and languages.69,70 In addition to differences across language, a study reported that more than half of the most commonly used cancer PROs do not meet plain-language best practices.71 Differences across language and low health literacy, coupled with the nonadherence to plain-language practices in PRO development, result in the risk of data loss. However, well-validated patient-report measures that are translated into diverse languages can be pooled to analyze the data from multinational clinical research and provide reliable symptom assessment.72 Increasingly, comparable fatigue PRO data support a standardized use of well-defined terminology based on professional consensus and guidelines.
Fatigue PROs
While instruments to evaluate CRF PROs are numerous, we summarize selected measures most widely reported in the literature. Tables 1-373-144 outline the contexts in which researchers have tested these measures for reliability and the types of validity assessed. We excluded measures that were missing information for 2 or more of the categorical properties included within Tables 1, 2, and 3. These categories comprise validated populations, translation, cultural adaptations, number of items, constructs measured, response options, clinically relevant cutoff scores, estimated completion times, and practical information about licensing fees and format. The measures represented in Tables 1, 2, and 3 have been used across multiple countries throughout North America, South America, Europe, the Middle East, Africa, and Asia, and they are organized according to clinical and research purposes. Given that screening would be the first step in determining the presence or severity of CRF, measures that serve this purpose are listed first. The measures that support further evaluation when screening results are moderate or severe are listed next.
TABLE 1. Category 1 Validated Patient-Reported Cancer-Related Fatigue Assessment Instruments
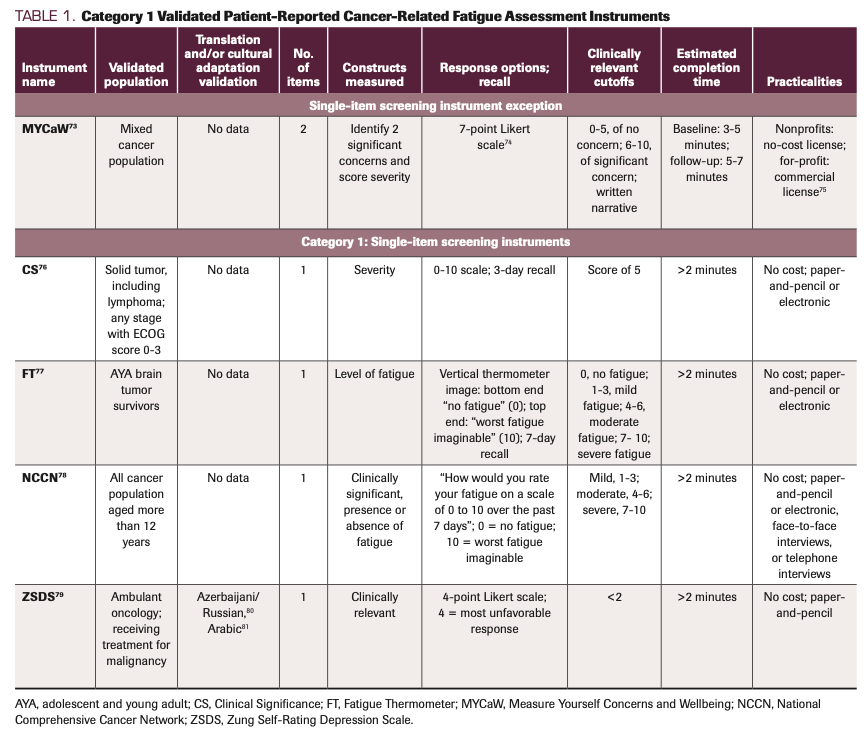
TABLE 2. Category 2 Validated Patient-Reported Cancer-Related Fatigue Assessment Instruments
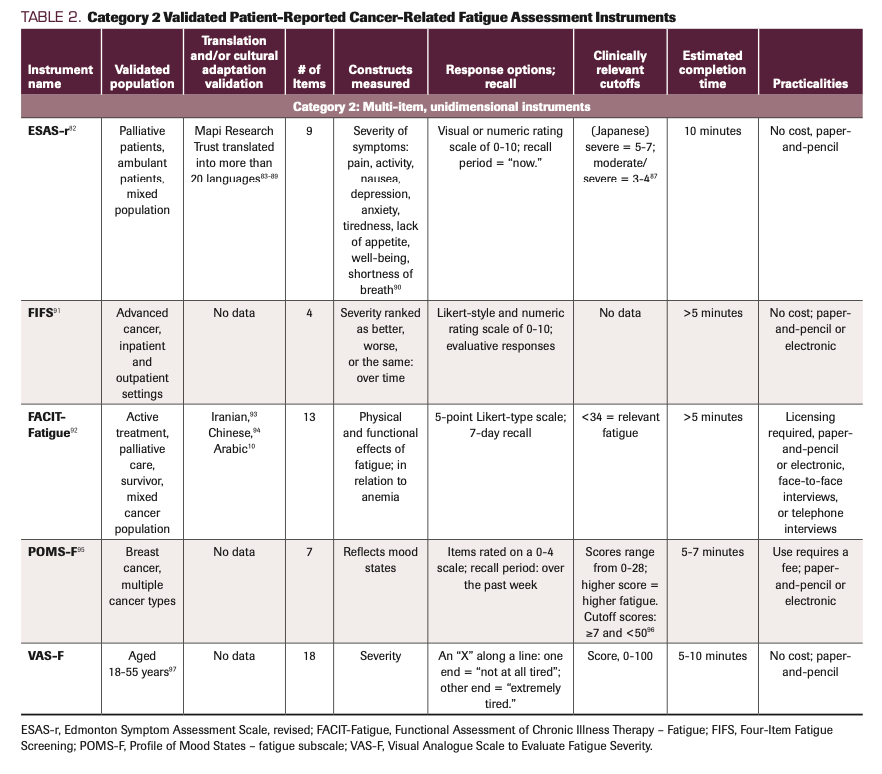
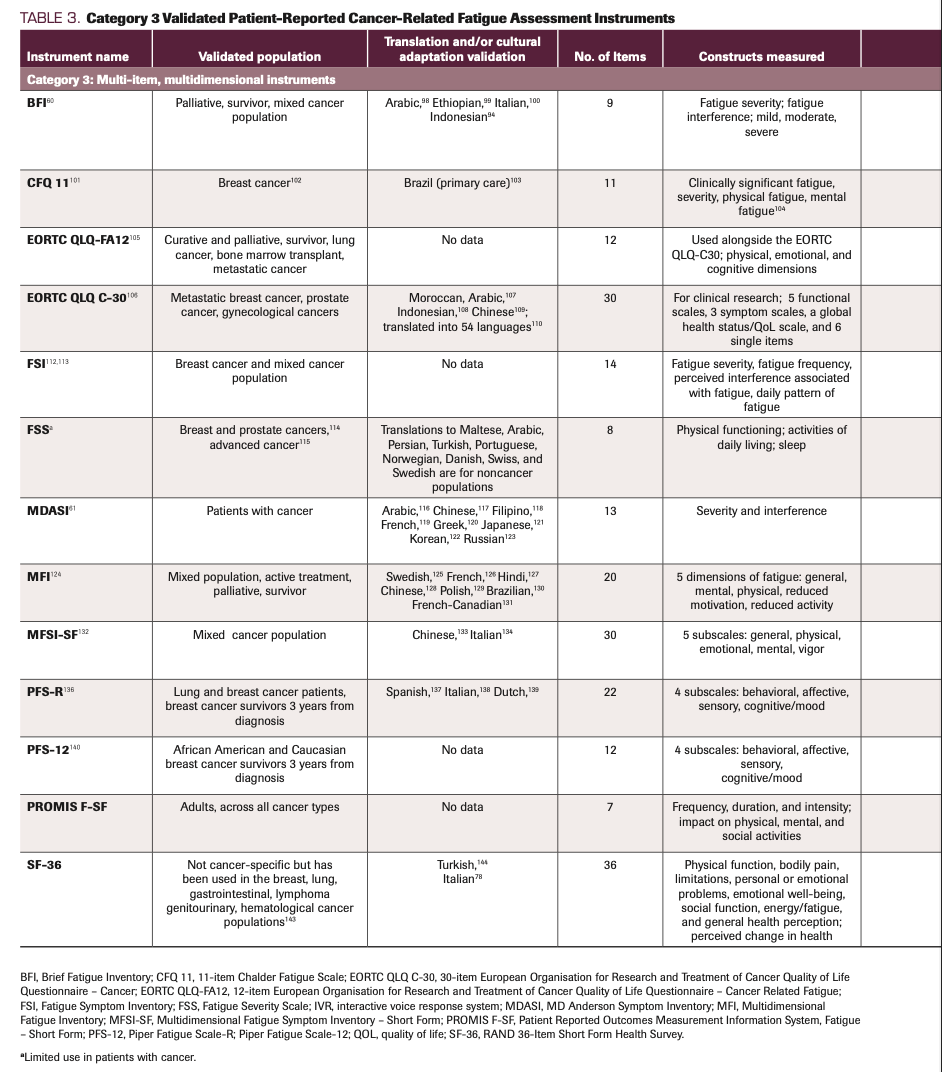
TABLE 3. Category 3 Validated Patient-Reported Cancer-Related Fatigue Assessment Instruments
The validated measures included in Tables 1, 2, and 3 are grouped based on common characteristics and are divided into 3 categories. Category 1 (Table 1) includes measures for screening, category 2 (Table 2) includes unidimensional measures, and category 3 (Table 3) includes multidimensional measures. The categorization has 1 exception: We present the Measure Yourself Concerns and Wellbeing (MYCaW) measure on its own due to its unique properties. An outlier of the screening group, the MYCaW instrument145 screener asks the patient to report their most important concerns in their own words. Similar to assessments found in the UK eHealth care agenda, an advantage of the MYCaW scale is that it empowers patients to participate in their own health by specifying fatigue/weakness as 1 of the 2 concerns and then assessing the extent that the fatigue bothers them on a 0- to 6-point scale. This scale highlights the patient’s subjective interpretation of the impact of fatigue. A drawback to the MYCaW approach is the difficulty of analyzing heterogeneously reported symptoms.
Single-item measures
Category 1 measures used for screening take no more than 2 minutes to complete. Measures in this category include Clinical Significance, Fatigue Thermometer, and the Zung Self-Rating Depression Scale. Studies have validated the Clinical Significance tool in patients diagnosed with a solid tumor, including lymphoma, at any stage with an ECOG score of 0 to 3; the Fatigue Thermometer has been validated in adolescent and young adult survivors of brain tumors. The NCCN recommends use of a single-item screener question to screen patients for fatigue at regular intervals using age-appropriate measures.1 Kirsh et al79 validated the single fatigue item of the Zung Self-Rating Depression Scale in ambulatory oncology patients undergoing treatment for malignancy. The overall benefit of single-item screening measures is that they offer quick, clinically meaningful cutoffs to determine if further evaluation is warranted. However, it is essential to not rely on these measures in isolation. They do not provide a thorough assessment of CRF across the physical, emotional, and cognitive domains of interest. Additionally, there may not be well-established clinical cutoff scores with known sensitivity and specificity in a given population.
Multi-item, unidimensional instruments
Category 2 includes multi-item, unidimensional instruments that are well known and often used, such as the Functional Assessment of Chronic Illness Therapy – Fatigue, and the Profile of Mood States – Fatigue subscale (POMS-F). These instruments contain multiple items designed to measure 1 CRF dimension. The ESAS-r independently measures a range of symptoms, one being fatigue or tiredness. The Four Item Fatigue Scale (FIFS) has the least number of items in this category and therefore has the least patient burden. Researchers found the FIFS to have concurrent validity with the BFI in the context of changes in CRF over time and severity in patients with advanced cancer being treated in palliative medicine; however, the FIFS requires further test-retest reliability.91 With 7 items, the POMS-F instrument has the second-least number. Like the FIFS, the POMS-F is exceptional for reducing patient burden and has been validated in breast cancer patients.96 The benefits of these multi-item, unidimensional tools are that they use multiple items to assess CRF while demonstrating reliability and validity in relation to other known measures. Despite these benefits, however, unidimensional instruments can be limiting, because the scope of measurement is only the physical impact of fatigue, excluding its emotional and cognitive impacts.146
Multi-item, multidimensional instruments
Category 3 includes multi-item, multidimensional instruments used for assessing CRF. They are multidimensional in that each measure combines a variety of dimensions known to characterize CRF in different ways. The dimensions measured include physical, emotional, cognitive, impact on functioning, severity, interference, HRQOL, vigor, associated symptoms, and patterns of fatigue. BFI is a category 3 instrument that clinicians and researchers commonly use. In contrast to category 1 and 2 instruments, those in category 3 provide a more thorough assessment of the patient experience of CRF, given its multidimensional measures. For example, in category 3, the 30-item European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Cancer (EORTC QLQ C-30), fatigue severity is assessed with items No. 12 (“Have you felt weak?”) and No. 18 (“Were you tired?”), with scores ranging from 0 to 100. With its 1-week perspective, the EORTC QLQ C-30 significantly adds to the 24-hour score findings of the ESAS-r while being more patient centered and less symptom oriented. Another advantage of the EORTC QLQ C-30 is that it includes symptom scales (including fatigue) as well as functional scales. The inclusion of symptom and functional scales allows clinicians and researchers to see trends across dimensions, which allows for greater understanding of CRF in association with other symptoms and QOL concerns in and across different scales. Furthermore, multidimensional instruments help inform interventional studies. Findings from these instruments provide in-depth data regarding changes in severity and the dimensions most impacted by the intervention.
It can be argued that despite the additional data that category 3 measurements provide, they may fail to provide evidence for the variability that can occur across these dimensions and constructs of CRF. Another potential disadvantage to using multidimensional scales is their length. The longest category 3 instruments include 36 items, and this length can be burdensome for fatigued patients no matter what their disease stage. In addition, scoring larger instruments by hand can be demanding for clinicians. When deciding on a measure to use, it is essential to consider all these factors and their impact on the study objectives.
Proposals for Standardization
Based on the available results of psychometric and population-based studies, we recommend the following PROs for measuring CRF (Table 4): The NCCN single-item tool is recommended for measuring clinical significance, and the BFI offers fast, easy, helpful cutoffs and measures of both severity and daily functioning interference. Further, when assessing multidimensional qualities and fatigue symptom cluster is necessary, either the MDASI or ESAS-r offers a robust CRF measure. Furthermore, psychometrically rigorous translations of the MDASI into various non-English versions can be used to gather symptom severity and interference ratings that can be comparably interpreted across patient nationalities.72 The rigorously developed Patient Reported Outcomes Measurement Information System (PROMIS) measurement system, including the PROMIS–Fatigue Short Form, has advantages related to comparison and norms across populations. The MYCaW has been used in specialized cases73-75, 145 and studies have demonstrated that this tool is easy to implement in real-life clinical practice, and like the ESAS-r, offers multiple time perspectives, and complement each other by viewing CRF multidimensionally. Results of the EORTC QLQ-C30 are a good outcome measure for QOL during certain time points—such as pre- and post therapy—and are good for comparative research; however, they are limited in terms of longitudinal fatigue assessment.
TABLE 4. CRF PRO Recommendations
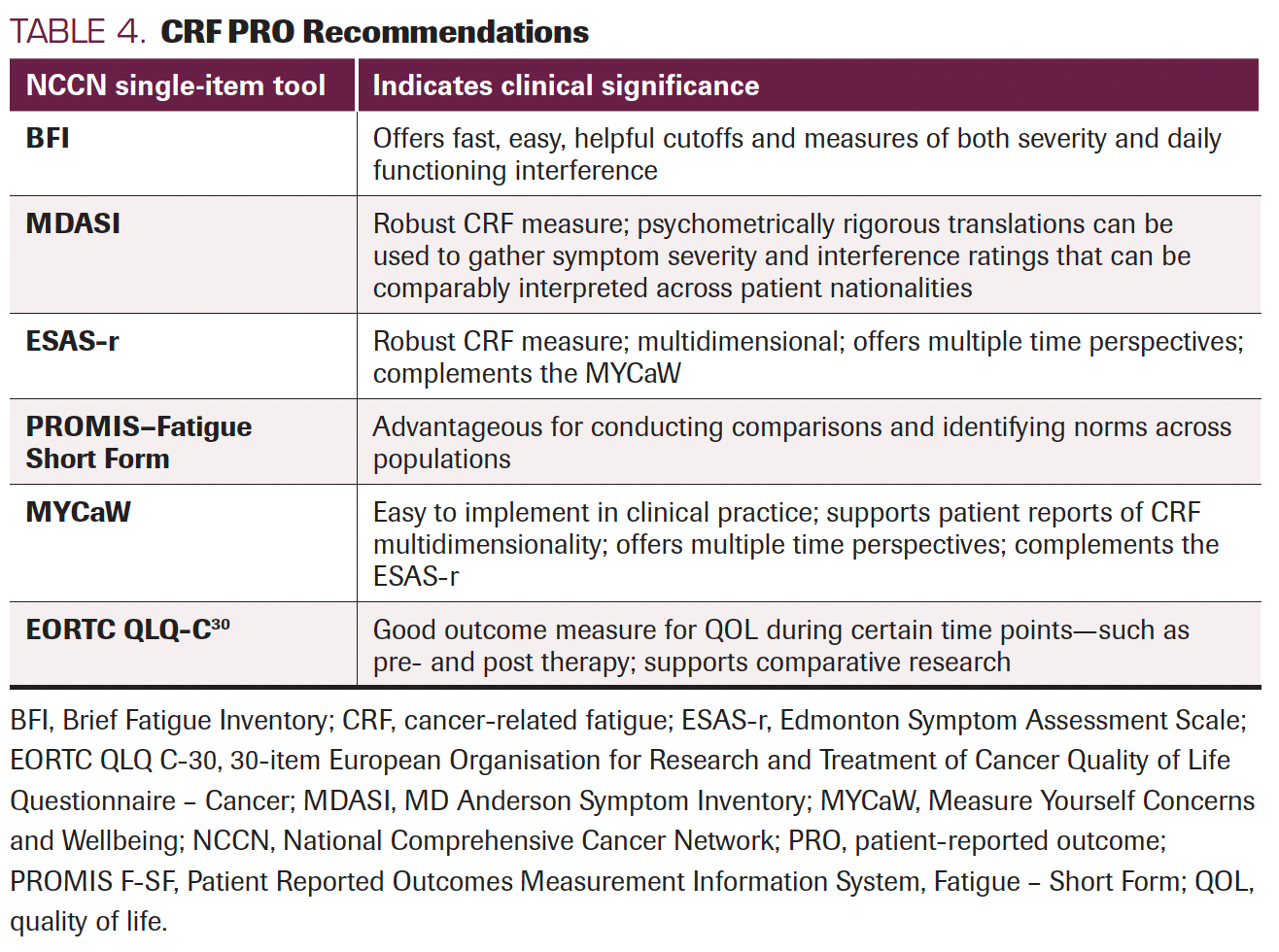
Complementary measurement use occurs when clinicians find it important to combine a symptom perspective (eg, ESAS-r) and a patient-centered perspective (eg, MYCaW) while assessing impacts to QOL (eg, EORTC QLQ-C30). While single-item screening measures are quick, they are limited in assessing the multiple domains of CRF yet completing longer instruments may be problematic and burdensome for patients with advanced cancer.
As we have shown, when the multidimensional nature of CRF is considered in addition to concerns such as recall period, treatment regimen, and the availability of validated measures that have been translated, selecting subjective CRF measures can become complex. This creates barriers for clinicians who attempt to integrate CRF into their decision-making and instead are faced with a lack of meaningful, interpretable summary measures.59 For these reasons, we suggest the following areas in which future research can help improve the use of PROs and our understanding of CRF.
Future Directions
Future research should explore several areas of CRF measurement and PRO use. From a patient perspective, the CRF assessment should include measurements across diverse populations and consider patient burden. The National Cancer Institute has recently implemented the Patient-Reported Outcomes Common Terminology Criteria for Adverse Events (PRO-CTCAE), which addresses this consideration. This recently validated PRO instrument is for clinical trial use in reporting symptomatic adverse events (AEs); it minimizes patient burden by shortening HRQOL questionnaires.147 In total, a library of 124 PRO-CTCAE items were created that represent 78 symptomatic toxicities from which researchers can choose the relevant items depending on the study population. The PRO-CTCAE was created to enhance the precision and patient-centeredness of AE reporting.148 Studies using the PRO-CTCAE library for CRF are beginning to emerge, suggesting this as a potential future direction for clinical trial research.
Additionally, researchers should evaluate patient preferences for handwritten vs electronic questionnaires. From a measurement consideration, researchers can explore completion times and completion rates for each of the validated fatigue PRO measures in real-world settings and how these may differ in electronic data collection compared with paper-and-pencil administration.
When considering measures in the design phase, researchers could include comparisons of instruments and where needed, provide cross-cultural validity to support international clinical guidelines.72 Additional research investigating patient preferences for specific CRF PRO instruments may find that patient preferences differ or align with researcher and clinician preferences. Future studies should also examine the impact of whether a questionnaire is free for public use or requires payment for licensing on the uptake of measures.
When undertaking CRF research, investigators should consider various methodologies that enrich the applicability and implementation of research outcomes in real-life integrative oncology (IO) clinical practice. For example, as in studies of supportive and palliative care, IO pragmatic studies need to respect the typical IO setting regarding patient preferences, patient-tailored treatment, and multimodal IO programs, as well as questions on how to maintain adherence and continuity of care. In doing so, pragmatic research will complement randomized control trials’ line of research.
CRF researchers should also consider broadening their focus—not just examining the impact of CRF on patients’ QOL, but also including additional oncology parameters. Such future studies could include measuring the impact of CRF on adherence to oncology treatment protocols149 and assessing the cost effectiveness of CRF analysis. Studies that explore CRF should examine its impact on, or association with, other symptoms, relationships, employment, and absenteeism/presenteeism; related outcomes should be included in studies when feasible.
These future research considerations increase the possibility for guidelines on CRF and IO to include rigorous pragmatic and controlled research in their inclusion criteria, even if not randomized, to address the real-life IO setting in leading cancer centers in the US and internationally. In addition, publications could include studies where CRF is the primary clinical outcome and studies that assess CRF-related QOL-concerns (eg, pain, insomnia, anxiety, appetite) in a broader patient-centered context. By recognizing these criteria, clinicians and researchers can better understand the overlap across the multidimensional aspects of fatigue.
Conclusions
This review introduced CRF, summarized the literature concerning CRF prevalence and impacts, challenges for diagnosis and treatment, issues with CRF measurement, the most widely reported CRF PRO measures, and provided recommendations on validated CRF PRO measures use based on specific contexts.
CRF is a complex and persistent symptom that impacts many patients with cancer throughout their disease experience. Like many symptoms, CRF is predominantly assessed through subjective PRO instruments for a variety of reasons across clinical practice and research. The more complex the measure and the more domains that are assessed, the greater the challenge of measuring CRF can become. As research progresses, it is increasingly evident that objective measures are needed to understand CRF etiology; such measures are instrumental in developing effective CRF interventions. In this effort, research utilizes objective lab- and performance-based measures, but it must be noted that objective measures, such as handgrip strength, lean body mass, and maximal inspiratory pressure, should not replace subjective measures.150 Rather, objective and subjective measures can work in a complementary way to support patient care.
Presenting the criteria for how researchers make measurement decisions in their published findings would further support a collective move toward a core group of measures that will improve the ability to compare clinical and research outcomes with one another. With this move toward standardization, we anticipate that our understanding of CRF and ways to effectively measure CRF will positively impact patient care with detailed guidelines that assist in improved CRF management and intervention.
Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
Author Affiliations
Danielle Gentile, PhD1*,2**; Dori Beeler, PhD1; Xin Shelley Wang, MD, MPH3; Eran Ben-Arye4; Suzanna Zick, ND, MPH5; Ting Bao, MD, DABMA, MS6,7; Linda E. Carlson, PhD8; Ricardo Ghelman, MD, PhD9,10; Viraj A. Master, MD, PhD, FACS11; Debu Tripathy, MD12; W. Iris Zhi, MD, PhD6
1Section of Research, Department of Supportive Oncology, Levine Cancer Institute, Atrium Health, Charlotte, NC, USA
2Cardinal Health Specialty Solutions, Dublin, OH, USA
3Department of Symptom Research, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
4Integrative Oncology Program, The Oncology Service; Lin, Carmel, and Zebulun Medical Centers, Clalit Health Services, Haifa, Israel; Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel
5Department of Family Medicine and Nutritional Sciences, University of Michigan, Ann Arbor, MI, USA
6Breast Medicine Service, Solid Tumor Division, Memorial Sloan Kettering Cancer Center, New York, NY, USA
7Integrative Medicine Service, Memorial Sloan Kettering Cancer Center, New York, NY, USA
8Division of Psychosocial Oncology, Department of Oncology, Cumming School of Medicine, University of Calgary, Alberta, Canada
9Department of Surgery, Laboratory of Medical Investigation, University of São Paulo Medical School, São Paulo, Brazil
10Brazilian Academic Consortium for Integrative Health – CABSIN, São Paulo, Brazil
11Department of Urology, Winship Cancer Institute, Emory University School of Medicine, Atlanta, GA, USA
12Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA
*Affiliation at time of research
**Current affiliation
References
- NCCN. Clinical Practice Guidelines in Oncology. Cancer-related fatigue, version 2.2020. Accessed December 2021. https://www.nccn.org/guidelines
- Schmidt ME, Hermann S, Arndt V, Steindorf K. Prevalence and severity of long-term physical, emotional, and cognitive fatigue across 15 different cancer entities. Cancer Med. 2020;9(21):8053-8061. doi:10.1002/cam4.3413
- Miller M, Kwekkeboom K, Cherwin C. The role of spirituality in symptom experiences among adults with cancer. Support Care Cancer. 2021;30(1):49-57. doi:10.1007/s00520-021-06399-z
- Zhi WI, Gentile D, Diller M, et al. Patient-reported outcomes of pain and related symptoms in integrative oncology practice and clinical research: evidence and recommendations. Oncology (Williston Park). 2021;35(1):35-41. doi:10.46883/onc.2021.3501.0035
- Hopkins J, Mumber MP. Patient navigation through the cancer care continuum: an overview. J Oncol Pract. 2009;5(4):150-152. doi:10.1200/JOP.0943501
- Narayanan V, Koshy C. Fatigue in cancer: a review of literature. Indian J Palliat Care. 2009;15(1):19-25. doi:10.4103/0973-1075.53507
- Aapro M, Scotte F, Bouillet T, Currow D, Vigano A. A practical approach to fatigue management in colorectal cancer. Clin Colorectal Cancer. 2017;16(4):275-285. doi:10.1016/j.clcc.2016.04.010
- Stone PC, Minton O. Cancer-related fatigue. Eur J Cancer. 2008;44(8):1097-1104. doi:10.1016/j.ejca.2008.02.037
- Ma Y, He B, Jiang M, et al. Prevalence and risk factors of cancer-related fatigue: a systematic review and meta-analysis. Int J Nurs Stud. 2020;111:103707. doi:10.1016/j.ijnurstu.2020.103707
- Al Maqbali M, Hughes C, Gracey J, Rankin J, Hacker E, Dunwoody L. Psychometric properties of the Arabic version of the Functional Assessment of Chronic Illnesses Therapy – Fatigue in Arabic cancer patients. J Pain Symptom Manage. 2020;59(1):130-138.e2. doi:10.1016/j.jpainsymman.2019.10.008
- Álvarez-Bustos A, de Pedro CG, Romero-Elías M, et al. Prevalence and correlates of cancer-related fatigue in breast cancer survivors. Support Care Cancer. 2021;29(11):6523-6534. doi:10.1007/s00520-021-06218-5
- Spathis A, Booth S, Grove S, Hatcher H, Kuhn I, Barclay S. Teenage and young adult cancer-related fatigue is prevalent, distressing, and neglected: it is time to intervene. a systematic literature review and narrative synthesis. J Adolesc Young Adult Oncol. 2015;4(1):3-17. doi:10.1089/jayao.2014.0023
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720-2726. doi:10.1001/jama.291.22.2720
- White MN, Dotan E, Catalano PJ, Cardin DB, Berlin JD. Advanced pancreatic cancer clinical trials: the continued underrepresentation of older patients. J Geriatr Oncol. 2019;10(4):540-546. doi:10.1016/j.jgo.2018.11.001
- Gupta D, Lis CG, Grutsch JF. The relationship between cancer-related fatigue and patient satisfaction with quality of life in cancer. J Pain Symptom Manage. 2007;34(1):40-47. doi:10.1016/j.jpainsymman.2006.10.012
- Jong MC, Boers I, Schouten van der Velden AP, et al. A randomized study of yoga for fatigue and quality of life in women with breast cancer undergoing (neo) adjuvant chemotherapy. J Altern Complement Med. 2018;24(9-10):942-953. doi:10.1089/acm.2018.0191
- Curt GA, Breitbart W, Cella D, et al. Impact of cancer‐related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5(5):353-360. doi:10.1634/theoncologist.5-5-353
- Lundberg PC, Rattanasuwan O. Experiences of fatigue and self-management of Thai Buddhist cancer patients undergoing radiation therapy. Cancer Nurs. 2007;30(2):146-155. doi:10.1097/01.NCC.0000265005.02559.43
- Wondie Y, Mehnert A, Hinz A. The Hospital Anxiety and Depression Scale (HADS) applied to Ethiopian cancer patients. PLoS One. 2020;15(12):e0243357. doi:10.1371/journal.pone.0243357
- Grossoehme DH, Friebert S, Baker JN, et al. Association of religious and spiritual factors with patient-reported outcomes of anxiety, depressive symptoms, fatigue, and pain interference among adolescents and young adults with cancer. JAMA Netw Open. 2020;3(6):e206696. doi:10.1001/jamanetworkopen.2020.6696
- Mortimer JE, Barsevick AM, Bennett CL, et al. Studying cancer-related fatigue: report of the NCCN scientific research committee. J Natl Compr Canc Netw. 2010;8(12):1331-1339. doi:10.6004/jnccn.2010.0101
- Weis J. Cancer-related fatigue: prevalence, assessment and treatment strategies. Expert Rev Pharmacoecon Outcomes Res. 2011;11(4):441-446. doi:10.1586/erp.11.44
- Hiensch AE, Bolam KA, Mijwel S, May AM, Wengström Y. Sense of coherence and its relationship to participation, cancer-related fatigue, symptom burden, and quality of life in women with breast cancer participating in the OptiTrain exercise trial. Support Care Cancer. 2020;28(11):5371-5379. doi:10.1007/s00520-020-05378-0
- Eriksson M, Lindström B. Antonovsky’s sense of coherence scale and its relation with quality of life: a systematic review. J Epidemiol Community Health. 2007;61(11):938-944. doi:10.1136/jech.2006.056028
- Barsevick AM, Cleeland CS, Manning DC, et al; ASCPRO (Assessing Symptoms of Cancer Using Patient-Reported Outcomes). ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. J Pain Symptom Manage. 2010;39(6):1086-1099. doi:10.1016/j.jpainsymman.2010.02.006
- Fabi A, Bhargava R, Fatigoni S, et al; ESMO Guidelines Committee. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol. 2020;31(6):713-723. doi:10.1016/j.annonc.2020.02.016
- Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3(7):961-968. doi:10.1001/jamaoncol.2016.6914
- Chow R, Bruera E, Sanatani M, et al. Cancer-related fatigue-pharmacological interventions: systematic review and network meta-analysis. BMJ Support Palliat Care. Published online September 30, 2021. doi:10.1136/bmjspcare-2021-003244
- Arring NM, Barton DL, Brooks T, Zick SM. Integrative therapies for cancer-related fatigue. Cancer J. 2019;25(5):349-356. doi:10.1097/PPO.0000000000000396
- Sadja J, Mills PJ. Effects of yoga interventions on fatigue in cancer patients and survivors: a systematic review of randomized controlled trials. Explore (NY). 2013;9(4):232-243. doi:10.1016/j.explore.2013.04.005
- Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. The Cochrane Database Syst Rev. 2017;1(1):CD010802. doi:10.1002/14651858.CD010802.pub2
- Wu C, Zheng Y, Duan Y, et al. Nonpharmacological interventions for cancer-related fatigue: a systematic review and Bayesian network meta-analysis. Worldviews Evid Based Nurs. 2019;16(2):102-110. doi:10.1111/wvn.12352
- Xiang Y, Lu L, Chen X, Wen Z. Does tai chi relieve fatigue? a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2017;12(4):e0174872. doi:10.1371/journal.pone.0174872
- Tang Y, Fu F, Gao H, Shen L, Chi I, Bai Z. Art therapy for anxiety, depression, and fatigue in females with breast cancer: a systematic review. J Psychosoc Oncol. 2019;37(1):79-95. doi:10.1080/07347332.2018.1506855
- Xie C, Dong B, Wang L, et al. Mindfulness-based stress reduction can alleviate cancer-related fatigue: a meta-analysis. J Psychosom Res. 2020;130:109916. doi:10.1016/j.jpsychores.2019.109916
- Paschoin de Oliveira Campos M, Riechelmann R, Martins LC, Hassan BJ, Assunção Casa FB, Del Giglio A. Guarana (Paullinia cupana) improves fatigue in breast cancer patients undergoing systemic chemotherapy. J Altern Complement Med. 2011;17(6):505-512. doi:10.1089/acm.2010.0571
- Braz del Giglio A, de Iracema Gomes Cubero D, Goberstein Lerner T, et al. Purified dry extract of Paullinia cupana (guaraná) (PC-18) for chemotherapy-related fatigue in patients with solid tumors: an early discontinuation study. J Diet Suppl. 2013;10(4):325-334. doi:10.3109/19390211.2013.830676
- Vaz de Melo Sette C, Bonaparte Ribas de Alcântara B, Maselli Schoueri JH, et al. Purified dry paullinia cupana (PC-18) extract for chemotherapy-induced fatigue: results of two double-blind randomized clinical trials. J Diet Suppl. 2018;15(5):673-683. doi:10.1080/19390211.2017.1384781
- Carvalho Lopes de Paula L, Fonseca F, Perazzo F, et al. Uncaria tomentosa (cat’s claw) improves quality of life in patients with advanced solid tumors. J Altern Complement Med. 2015;21(1):22-30. doi:10.1089/acm.2014.0127
- Bock PR, Hanisch J, Matthes H, Zänker KS. Targeting inflammation in cancer-related-fatigue: a rationale for mistletoe therapy as supportive care in colorectal cancer patients. Inflamm Allergy Drug Targets. 2014;13(2):105-111. doi:10.2174/1871528113666140428103332
- Li Oei S, Thronicke A, Kröz M, von Trott P, Schad F, Matthes H. Impact of oncological therapy and Viscum album L treatment on cancer-related fatigue and internal coherence in nonmetastasized breast cancer patients. Integr Cancer Ther. 2020;19:1534735420917211. doi:10.1177/1534735420917211
- Arring NM, Millstine D, Marks LA, Nail LM. Ginseng as a treatment for fatigue: a systematic review. J Altern Complement Med. 2018;24(7):624-633. doi:10.1089/acm.2017.0361
- Sadeghian M, Rahmani S, Zendehdel M, Hosseini SA, Zare Javid A. Ginseng and cancer-related fatigue: a systematic review of clinical trials. Nutr Cancer. 2021;73(8):1270-1281. doi:10.1080/01635581.2020.1795691
- Xu Y, Wang XS, Chen Y, Shi Q, Hsuan Chen T, Li P. A phase II randomized controlled trial of renshen yangrong tang herbal extract granules for fatigue reduction in cancer survivors. J Pain Symptom Manage. 2020;59(5):966-973. doi:10.1016/j.jpainsymman.2019.10.018
- Ben-Arye E, Dahan O, Shalom-Sharabi I, Samuels N. Inverse relationship between reduced fatigue and severity of anemia in oncology patients treated with integrative medicine: understanding the paradox. Support Care Cancer. 2018;26(12):4039-4048. doi:10.1007/s00520-018-4271-5
- Ben-Arye E, Samuels N, Schiff E, Gressel Raz O, Shalom Sharabi I, Lavie O. Quality-of-life outcomes in patients with gynecologic cancer referred to integrative oncology treatment during chemotherapy. Support Care Cancer. 2015;23(12):3411-3419. doi:10.1007/s00520-015-2690-0
- Shalom Sharabi I, Levin A, Schiff E, et al. Quality of life–related outcomes from a patient-tailored integrative medicine program: experience of Russian-speaking patients with cancer in Israel. Support Care Cancer. 2016;24(10):4345-4355. doi:10.1007/s00520-016-3274-3
- Saligan LN, Olson K, Filler K, et al; Multinational Association of Supportive Care in Cancer Fatigue Study Group – Biomarker Working Group. The biology of cancer-related fatigue: a review of the literature. Support Care Cancer. 2015;23(8):2461-2478. doi:10.1007/s00520-015-2763-0
- O’Higgins CM, Brady B, O’Connor B, Walsh D, Reilly RB. The pathophysiology of cancer-related fatigue: current controversies. Support Care Cancer. 2018;26(10):3353-3364. doi:10.1007/s00520-018-4318-7
- Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer‐related fatigue. Oncologist. 2007;12 Suppl 1:22-34. doi:10.1634/theoncologist.12-s1-22
- Wang X-S. Cancer-related fatigue. In: Berger AM, Shuster JL Jr, Von Roenn JH, eds. Principles and Practice of Palliative Care and Supportive Oncology. 4th ed. Wolters Kluwer/Lippincott Williams & Wilkins; 2013:84-95.
- Zick SM, Zwickey H, Wood L, et al. Preliminary differences in peripheral immune markers and brain metabolites between fatigued and non-fatigued breast cancer survivors: a pilot study. Brain Imaging Behav. 2014;8(4):506-516. doi:10.1007/s11682-013-9270-z
- Hampson JP, Zick SM, Khabir T, Wright BD, Harris RE. Altered resting brain connectivity in persistent cancer related fatigue. Neuroimage Clin. 2015;8:305-313. doi:10.1016/j.nicl.2015.04.022
- Bower JE. The role of neuro-immune interactions in cancer-related fatigue: biobehavioral risk factors and mechanisms. Cancer. 2019;125(3):353-364. doi:10.1002/cncr.31790
- Raudonis BM, Kelley IH, Rowe N, Ellis J. A pilot study of proinflammatory cytokines and fatigue in women with breast cancer during chemotherapy. Cancer Nurs. 2017;40(4):323-331. doi:10.1097/NCC.0000000000000406
- Crosswell AD, Lockwood KG, Ganz PA, Bower JE. Low heart rate variability and cancer-related fatigue in breast cancer survivors. Psychoneuroendocrinology. 2014;45:58-66. doi:10.1016/j.psyneuen.2014.03.011
- Fagundes CP, Murray DM, Seuk Hwang B, et al. Sympathetic and parasympathetic activity in cancer-related fatigue: more evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology. 2011;36(8):1137-1147. doi:10.1016/j.psyneuen.2011.02.005
- Kisiel-Sajewicz K, Davis MP, Siemionow V, et al. Lack of muscle contractile property changes at the time of perceived physical exhaustion suggests central mechanisms contributing to early motor task failure in patients with cancer-related fatigue. J Pain Symptom Manage. 2012;44(3):351-361. doi:10.1016/j.jpainsymman.2011.08.007
- Lai J-S, Crane PK, Cella D. Factor analysis techniques for assessing sufficient unidimensionality of cancer related fatigue. Qual Life Res. 2006;15(7):1179-1190. doi:10.1007/s11136-006-0060-6
- Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186-1196. doi:10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n
- Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634-1646. doi:10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v
- Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6-9.
- Wang XS, Zhao F, Fisch MJ, et al. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120(3):425-432. doi:10.1002/cncr.28434
- Luckett T, King MT. Choosing patient-reported outcome measures for cancer clinical research--practical principles and an algorithm to assist non-specialist researchers. Eur J Cancer. 2010;46(18):3149-3157. doi:10.1016/j.ejca.2010.08.002
- Howard GS, Ralph KM, Gulanick NA, Maxwell SE, Nance DW, Gerber SK. Internal invalidity in pretest–posttest self-report evaluations and a re-evaluation of retrospective pretests. Applied Psychological Measurement. 1979;3(1):1-23. doi:10.1177/014662167900300101
- Visser MR, Smets EM, Sprangers MA, de Haes HJ. How response shift may affect the measurement of change in fatigue. J Pain Symptom Manage. 2000;20(1):12-18. doi:10.1016/s0885-3924(00)00148-2
- Gerber LH. Cancer-related fatigue: persistent, pervasive, and problematic. Phys Med Rehabil Clin N Am. 2017;28(1):65-88. doi:10.1016/j.pmr.2016.08.004
- Hauser K, Rybicki L, Walsh D. What’s in a name? word descriptors of cancer-related fatigue. Palliat Med. 2010;24(7):724-730. doi:10.1177/0269216310376557
- Radbruch L, Sabatowski R, Elsner F, Everts J, Mendoza T, Cleeland C. Validation of the German version of the Brief Fatigue Inventory. J Pain Symptom Manage. 2003;25(5):449-458. doi:10.1016/s0885-3924(03)00073-3
- Radbruch L, Strasser F, Elsner F, et al; Research Steering Committee of the European Association for Palliative Care (EAPC). Fatigue in palliative care patients — an EAPC approach. Palliat Med. 2008;22(1):13-32. doi:10.1177/0269216307085183
- Papadakos JK, Charow RC, Papadakos CJ, Moody LJ, Giuliani ME. Evaluating cancer patient-reported outcome measures: readability and implications for clinical use. Cancer. 2019;125(8):1350-1356. doi:10.1002/cncr.31928
- Wang XS, Cleeland CS, Mendoza TR, et al. Impact of cultural and linguistic factors on symptom reporting by patients with cancer. J Natl Cancer Inst. 2010;102(10):732-738. doi:10.1093/jnci/djq097
- Jolliffe R, Seers H, Jackson S, Caro E, Weeks L, Polley MJ. The responsiveness, content validity, and convergent validity of the Measure Yourself Concerns and Wellbeing (MYCaW) patient-reported outcome measure. Integr Cancer Ther. 2015;14(1):26-34. doi:10.1177/1534735414555809
- Frenkel M, Cohen L, Peterson N, Palmer JL, Swint K, Bruera E. Integrative medicine consultation service in a comprehensive cancer center: findings and outcomes. Integr Cancer Ther. 2010;9(3):276-283. doi:10.1177/1534735410378663
- Measure Yourself Concerns and Wellbeing (MYCaW). Meaningful Measures. Accessed March 30, 2021. https://www.meaningfulmeasures.co.uk/mycaw
- Butt Z, Wagner LI, Beaumont JL, et al. Use of a single-item screening tool to detect clinically significant fatigue, pain, distress, and anorexia in ambulatory cancer practice. J Pain Symptom Manage. 2008;35(1):20-30. doi:10.1016/j.jpainsymman.2007.02.040
- Brand SR, Chordas C, Liptak C, Manley P, Recklitis C. Screening for fatigue in adolescent and young adult pediatric brain tumor survivors: accuracy of a single-item screening measure. Support Care Cancer. 2016;24(8):3581-3587. doi:10.1007/s00520-016-3150-1
- Mosconi P, Cifani S, Crispino S, Fossati R, Apolone G. The performance of SF-36 Health Survey in patients with laryngeal cancer. Head and Neck Cancer Italian Working Group. Head Neck. 2000;22(2):175-182. doi:10.1002/(sici)1097-0347(200003)22:2<175::aid-hed10>3.0.co;2-v
- Kirsh KL, Passik S, Holtsclaw E, Donaghy K, Theobald D. I get tired for no reason: a single item screening for cancer-related fatigue. J Pain Symptom Manage. 2001;22(5):931-937. doi:10.1016/s0885-3924(01)00350-5
- Mammadova F, Sultanov M, Hajiyeva A, Aichberger M, Heinz A. Translation and adaptation of the Zung Self-Rating Depression Scale for application in the bilingual Azerbaijani population. Eur Psychiatry. 2012;27(Suppl 2):S27-S31. doi:10.1016/s0924-9338(12)75705-x
- Kirkby R, Al Saif A, el-din Mohamed G. Validation of an Arabic translation of the Zung Self-Rating Depression Scale. Ann Saudi Med. 2005;25(3):205-208. doi:10.5144/0256-4947.2005.205
- Ripamonti CI, Bandieri E, Pessi MA, Maruelli A, Buonaccorso L, Miccinesi G. The Edmonton Symptom Assessment System (ESAS) as a screening tool for depression and anxiety in non-advanced patients with solid or haematological malignancies on cure or follow-up. Support Care Cancer. 2014;22(3):783-793. doi:10.1007/s00520-013-2034-x
- Chinda M, Jaturapatporn D, Kirshen AJ, Udomsubpayakul U. Reliability and validity of a Thai version of the Edmonton Symptom Assessment Scale (ESAS-Thai). J Pain Symptom Manage. 2011;42(6):954-960. doi:10.1016/j.jpainsymman.2011.02.020
- Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manage. 2017;53(3):630-643. doi:10.1016/j.jpainsymman.2016.10.370
- Gretarsdottir H, Fridriksdottir N, Gunnarsdottir S. Psychometric properties of the Icelandic version of the revised Edmonton Symptom Assessment Scale. J Pain Symptom Manage. 2016;51(1):133-137. doi:10.1016/j.jpainsymman.2015.08.007
- Pautex S, Vayne-Bossert P, Bernard M, et al. Validation of the French version of the Edmonton Symptom Assessment System. J Pain Symptom Manage. 2017;54(5):721-726.e1. doi:10.1016/j.jpainsymman.2017.07.032
- Yamaguchi T, Morita T, Nitto A, et al. Establishing cutoff points for defining symptom severity using the Edmonton Symptom Assessment System–revised Japanese version. J Pain Symptom Manage. 2016;51(2):292-297. doi:10.1016/j.jpainsymman.2015.09.011
- Paiva CE, Manfredini LL, Paiva BS, Hui D, Bruera E. The Brazilian version of the Edmonton Symptom Assessment System (ESAS) is a feasible, valid and reliable instrument for the measurement of symptoms in advanced cancer patients. PLoS One. 2015;10(7):e0132073. doi:10.1371/journal.pone.0132073
- Carvajal A, Centeno C, Watson R, Bruera E. A comprehensive study of psychometric properties of the Edmonton Symptom Assessment System (ESAS) in Spanish advanced cancer patients. Eur J Cancer. 2011;47(12):1863-1872. doi:10.1016/j.ejca.2011.03.027
- Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88(9):2164-2171. doi:10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5
- Davis MP, Khoshknabi D, Walsh D, et al. Four-item fatigue screen: replacing the Brief Fatigue Index. Am J Hosp Palliat Care. 2013;30(7):652-656. doi:10.1177/1049909112460567
- Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74. doi:10.1016/s0885-3924(96)00274-6
- Meysami A, Yari H, Haghighat S, Montazeri A. The Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-Fatigue subscale): validity and reliability of the Iranian version. Oncol Res Treat. 2017;40(12):789-793. doi:10.1159/000479588
- Paramita N, Nusdwinuringtyas N, Nuhonni SA, et al. Validity and reliability of the Indonesian version of the Brief Fatigue Inventory in cancer patients. J Pain Symptom Manage. 2016;52(5):744-751. doi:10.1016/j.jpainsymman.2016.04.011
- Fisher MI, Davies C, Lacy H, Doherty D. Oncology Section EDGE Task Force on Cancer: measures of cancer-related fatigue—a systematic review. Rehabilitation Oncology. 2018;36(2):93-105. doi:10.1097/01.REO.0000000000000124
- Goedendorp MM, Jacobsen PB, Andrykowski MA. Fatigue screening in breast cancer patients: identifying likely cases of cancer-related fatigue. Psychooncology. 2016;25(3):275-281. doi:10.1002/pon.3907
- Visual Analogue Scale to Evaluate Fatigue Severity (VAS-F). In: Shahid A, Wilkinson K, Marcu S, Shapiro CM, eds. STOP, THAT and One Hundred Other Sleep Scales. Springer; 2011:399-402.
- Suleiman K, Al Kalaldeh M, AbuSharour L, et al. Validation study of the Arabic version of the Brief Fatigue Inventory (BFI-A). East Mediterr Health J. 2019;25(11):784-790. doi:10.26719/emhj.19.032
- Tekle Gebremariam G, Anshabo AT, Tigeneh W, Engidawork E. Validation of the Amharic version of the Brief Fatigue Inventory for Assessment of Cancer-Related Fatigue in Ethiopian cancer patients. J Pain Symptom Manage. 2018;56(2):264-272. doi:10.1016/j.jpainsymman.2018.04.015
- Catania G, Bell C, Ottonelli S, et al. Cancer-related fatigue in Italian cancer patients: validation of the Italian version of the Brief Fatigue Inventory (BFI). Support Care Cancer. 2013;21(2):413-419. doi:10.1007/s00520-012-1539-z
- Jackson C. The Chalder Fatigue Scale (CFQ 11). Occup Med (Lond). 2015;65(1):86. doi:10.1093/occmed/kqu168
- Hughes A, Suleman S, Rimes KA, Marsden J, Chalder T. Cancer-related fatigue and functional impairment – towards an understanding of cognitive and behavioural factors. J Psychosom Res. 2020;134:110127. doi:10.1016/j.jpsychores.2020.110127
- Cho HJ, Costa E, Rossi Menezes P, Chalder T, Bhugra D, Wessely S. Cross-cultural validation of the Chalder Fatigue Questionnaire in Brazilian primary care. J Psychosom Res. 2007;62(3):301-304. doi:10.1016/j.jpsychores.2006.10.018
- Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147-153. doi:10.1016/0022-3999(93)90081-p
- Weis J, Wirtz MA, Tomaszewski KA, et al; EORTC Quality of Life Group. Sensitivity to change of the EORTC quality of life module measuring cancer‐related fatigue (EORTC QLQ‐Fa12): results from the international psychometric validation. Psychooncology. 2019;28(8):1753-1761. doi:10.1002/pon.5151
- McLachlan SA, Devins GM, Goodwin PJ. Validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ-C30) as a measure of psychosocial function in breast cancer patients. Eur J Cancer. 1998;34(4):510-517. doi:10.1016/s0959-8049(97)10076-4
- Nejjari C, El Fakir S, Bendahhou K, et al. Translation and validation of European Organization for Research and treatment of Cancer Quality of Life Questionnaire – C30 into Moroccan version for cancer patients in Morocco. BMC Res Notes. 2014;7:228. doi:10.1186/1756-0500-7-228
- Perwitasari DA, Atthobari J, Dwiprahasto I, et al. Translation and validation of EORTC QLQ-C30 into Indonesian version for cancer patients in Indonesia. Jpn J Clin Oncol. 2011;41(4):519-529. doi:10.1093/jjco/hyq243
- Zhao H, Kanda K. Translation and validation of the standard Chinese version of the EORTC QLQ-C30. Qual Life Res. 2000;9(2):129-137. doi:10.1023/a:1008981520920
- Shih C-L, Chen C-H, Sheu C-F, Lang H-C, Hsieh C-L. Validating and improving the reliability of the EORTC QLQ-C30 using a multidimensional Rasch model. Value Health. 2013;16(5):848-854. doi:10.1016/j.jval.2013.05.004
- Snyder CF, Blackford AL, Brahmer JR, et al. Needs assessments can identify scores on HRQOL questionnaires that represent problems for patients: an illustration with the Supportive Care Needs Survey and the QLQ-C30. Qual Life Res. 2010;19(6):837-845. doi:10.1007/s11136-010-9636-2
- Traeger L, Braun IM, Greer JA, Temel JS, Cashavelly B, Pirl WF. Parsing depression from fatigue in patients with cancer using the Fatigue Symptom Inventory. J Pain Symptom Manage. 2011;42(1):52-59. doi:10.1016/j.jpainsymman.2010.10.262
- Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: further validation of the Fatigue Symptom Inventory. Qual Life Res. 2000;9(7):847-854. doi:10.1023/a:1008900413113
- Stone P, Richards M, A’Hern R, Hardy J. Fatigue in patients with cancers of the breast or prostate undergoing radical radiotherapy. J Pain Symptom Manage. 2001;22(6):1007-1015. doi:10.1016/s0885-3924(01)00361-x
- Stone P, Hardy J, Broadley K, Tookman AJ, Kurowska A, A’Hern R. Fatigue in advanced cancer: a prospective controlled cross-sectional study. Br J Cancer. 1999;79(9-10):1479-1486. doi:10.1038/sj.bjc.6690236
- Nejmi M, Wang XS, Mendoza TR, Gning I, Cleeland CS. Validation and application of the Arabic version of the M. D. Anderson symptom inventory in Moroccan patients with cancer. J Pain Symptom Manage. 2010;40(1):75-86. doi:10.1016/j.jpainsymman.2009.12.007
- Wang XS, Wang Y, Guo H, Mendoza TR, Hao X-S, Cleeland CS. Chinese version of the M. D. Anderson Symptom Inventory: validation and application of symptom measurement in cancer patients. Cancer. 2004;101(8):1890-1901. doi:10.1002/cncr.20448
- Wang XS, Laudico AV, Guo H, et al. Filipino version of the M. D. Anderson Symptom Inventory: validation and multisymptom measurement in cancer patients. J Pain Symptom Manage. 2006;31(6):542-552. doi:10.1016/j.jpainsymman.2005.11.011
- Guirimand F, Buyck J-F, Lauwers-Allot E, et al. Cancer-related symptom assessment in France: validation of the French M. D. Anderson Symptom Inventory. J Pain Symptom Manage. 2010;39(4):721-733. doi:10.1016/j.jpainsymman.2009.08.014
- Mystakidou K, Cleeland C, Tsilika E, et al. Greek M.D. Anderson Symptom Inventory: validation and utility in cancer patients. Oncology. 2004;67(3-4):203-210. doi:10.1159/000081318
- Okuyama T, Wang XS, Akechi T, et al. Japanese version of the MD Anderson Symptom Inventory: a validation study. J Pain Symptom Manage. 2003;26(6):1093-1104. doi:10.1016/j.jpainsymman.2003.05.003
- Yun YH, Mendoza TR, Kang IO, et al. Validation study of the Korean version of the M. D. Anderson Symptom Inventory. J Pain Symptom Manage. 2006;31(4):345-352. doi:10.1016/j.jpainsymman.2005.07.013
- Ivanova MO, Ionova TI, Kalyadina SA, et al. Cancer-related symptom assessment in Russia: validation and utility of the Russian M. D. Anderson Symptom Inventory. J Pain Symptom Manage. 2005;30(5):443-453. doi:10.1016/j.jpainsymman.2005.04.015
- Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315-325. doi:10.1016/0022-3999(94)00125-o
- Lundh Hagelin C, Wengström Y, Runesdotter S, Fürst CJ. The psychometric properties of the Swedish Multidimensional Fatigue Inventory MFI-20 in four different populations. Acta Oncol. 2007;46(1):97-104. doi:10.1080/02841860601009430
- Gentile S, Delarozière JC, Favre F, Sambuc R, San Marco JL. Validation of the French ‘multidimensional fatigue inventory’ (MFI 20). Eur J Cancer Care (Engl). 2003;12(1):58-64. doi:10.1046/j.1365-2354.2003.00295.x
- Chandel P, Sultan A, Khan KA, Choudhary V, Parganiha A. Validation of the Hindi version of the Multidimensional Fatigue Inventory-20 (MFI-20) in Indian cancer patients. Support Care Cancer. 2015;23(10):2957-2964. doi:10.1007/s00520-015-2661-5
- Tian J, Hong J-S. Validation of the Chinese version of Multidimensional Fatigue Inventory-20 in Chinese patients with cancer. Support Care Cancer. 2012;20(10):2379-2383. doi:10.1007/s00520-011-1357-8
- Buss T, Kruk A, Wiśniewski P, Modlinska A, Janiszewska J, Lichodziejewska-Niemierko M. Psychometric properties of the Polish version of the Multidimensional Fatigue Inventory-20 in cancer patients. J Pain Symptom Manage. 2014;48(4):730-737. doi:10.1016/j.jpainsymman.2013.11.015
- Baptista RLR, Biasoli I, Scheliga A, et al. Psychometric properties of the Multidimensional Fatigue Inventory in Brazilian Hodgkin’s lymphoma survivors. J Pain Symptom Manage. 2012;44(6):908-915. doi:10.1016/j.jpainsymman.2011.12.275
- Fillion L, Gélinas C, Simard S, Savard J, Gagnon P. Validation evidence for the French Canadian adaptation of the Multidimensional Fatigue Inventory as a measure of cancer-related fatigue. Cancer Nurs. 2003;26(2):143-154. doi:10.1097/00002820-200304000-00008
- Donovan K, Stein K, Lee M, Leach C, Ilozumba O, Jacobsen P. Systematic review of the Multidimensional Fatigue Symptom Inventory-Short Form. Support Care Cancer. 2015;23(1):191-212. doi: 10.1007/s00520-014-2389-7
- Chan A, Lew C, Wang XJ, et al. Psychometric properties and measurement equivalence of the Multidimensional Fatigue Syndrome Inventory – Short Form (MFSI-SF) amongst breast cancer and lymphoma patients in Singapore. Health Qual Life Outcomes. 2018;16(1):20. doi:10.1186/s12955-018-0846-6
- Pino MS, Cheli S, Martella F, Melidei C, Caligiani L, Fioretto L. A preliminary validation of an Italian version of the Multidimensional Fatigue Symptom Inventory – Short Form (MFSI-SF-I). Ann Oncology. 2015;26(Suppl 6):VI116. doi:10.1093/annonc/mdv346.10
- Liu L, Fiorentino L, Natarajan L, et al. Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psychooncology. 2009;18(2):187-194. doi:10.1002/pon.1412
- Piper BF, Dibble SL, Dodd MJ, et al. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncology nursing forum. 1998; 25(4):677-684.
- Cantarero-Villanueva I, Fernández-Lao C, Díaz-Rodríguez L, et al. The Piper Fatigue Scale –Revised: translation and psychometric evaluation in Spanish-speaking breast cancer survivors. Qual Life Res. 2014;23(1):271-276. doi:10.1007/s11136-013-0434-5
- Annunziata MA, Muzzatti B, Mella S, et al. The revised Piper Fatigue Scale (PFS-R) for Italian cancer patients: a validation study. Tumori. 2010;96(2):276-281.
- Dagnelie PC, Pijls-Johannesma MCG, Pijpe A, et al. Psychometric properties of the revised Piper Fatigue Scale in Dutch cancer patients were satisfactory. J Clin Epidemiol. 2006;59(6):642-649. doi:10.1016/j.jclinepi.2005.09.015
- Reeve BB, Stover AM, Alfano CM, et al. The Piper Fatigue Scale-12 (PFS-12): psychometric findings and item reduction in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2012;136(1):9-20. doi:10.1007/s10549-012-2212-4
- Stover AM, Reeve BB, Piper BF, et al. Deriving clinically meaningful cut-scores for fatigue in a cohort of breast cancer survivors: a Health, Eating, Activity, and Lifestyle (HEAL) study. Qual Life Res. 2013;22(9):2279-2292. doi:10.1007/s11136-013-0360-6
- Ameringer S, Elswick RK Jr, Menzies V, et al. Psychometric evaluation of the Patient-Reported Outcomes Measurement Information System Fatigue – Short Form across diverse populations. Nurs Res. 2016;65(4):279-289. doi:10.1097/NNR.0000000000000162
- Brown LF, Kroenke K, Theobald DE, Wu J. Comparison of SF-36 vitality scale and Fatigue Symptom Inventory in assessing cancer-related fatigue. Support Care Cancer. 2011;19(8):1255-1259. doi:10.1007/s00520-011-1148-2
- Pinar R. Reliability and construct validity of the SF-36 in Turkish cancer patients. Qual Life Res. 2005;14(1):259-264. doi:10.1007/s11136-004-2393-3
- Paterson C, Thomas K, Manasse A, Cooke H, Peace G. Measure Yourself Concerns and Wellbeing (MYCaW): an individualised questionnaire for evaluating outcome in cancer support care that includes complementary therapies. Complement Ther Med. 2007;15(1):38-45. doi:10.1016/j.ctim.2006.03.006
- Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol. 2009;20(1):17-25. doi:10.1093/annonc/mdn537
- Taarnhøj GA, Kennedy FR, Absolom KL, et al. Comparison of EORTC QLQ-C30 and PRO-CTCAE™ questionnaires on six symptom items. J Pain Symptom Manage. 2018;56(3):421-429. doi:10.1016/j.jpainsymman.2018.05.017
- Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(9):dju244. doi:10.1093/jnci/dju244
- Shalom-Sharabi I, Lavie O, Samuels N, Keinan-Boker L, Lev E, Ben-Arye E. Can complementary medicine increase adherence to chemotherapy dosing protocol? a controlled study in an integrative oncology setting. J Cancer Res Clin Oncol. 2017;143(12):2535-2543. doi:10.1007/s00432-017-2509-0
- Schvartsman G, Park M, Liu DD, Yennu S, Bruera E, Hui D. Could objective tests be used to measure fatigue in patients with advanced cancer? J Pain Symptom Manage. 2017;54(2):237-244. doi:10.1016/j.jpainsymman.2016.12.343

Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.
Late Hepatic Recurrence From Granulosa Cell Tumor: A Case Report
Granulosa cell tumors exhibit late recurrence and rare hepatic metastasis, emphasizing the need for lifelong surveillance in affected patients.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.