Marked Antitumor Activity Produced by Rituximab Plus Thalidomide in Mantle Cell Lymphoma
This special supplement to Oncology News International includes updated results of studies with anti-CD20 therapy and other targeted therapies in the treatment of lymphomas, chronic lymphocytic leukemia, and immune thrombocytopenic purpura. The results were presented at the American Society of Hematology 44th Annual Meeting in Philadelphia, December 6 to 10, 2002.
VIENNA-Mantle cell lymphoma (MCL) is often refractory to standard chemotherapy, and there are few alternative therapeutic opportunities or strategies. Thalidomide (Thalomid) has demonstrated antitumor activity in multiple myeloma and is being investigated for several other malignancies, including prostate cancer, glioma, and mantle cell lymphoma.[1] Johannes Drach, MD, and colleagues at University Hospital in Vienna, Austria, presented data from a phase II trial evaluating the efficacy and safety of rituximab (Rituxan) combined with thalidomide in patients with mantle cell lymphoma that did not respond to or relapsed after treatment with standard CHOP (cyclophosphamide, doxorubicin, vincristine (Oncovin), and prednisone) or CHOP-like chemothera py (ASH abstract 606).[2] Patients received rituximab 375 mg/m2/day on days 1, 8, 15, and 22. Thalidomide was administered orally at 200 mg/day on day 1, followed by a dose escalation to 400 mg/day on day 15, and continued daily as maintenance therapy until disease progression, disease relapse, or unacceptable toxicity.
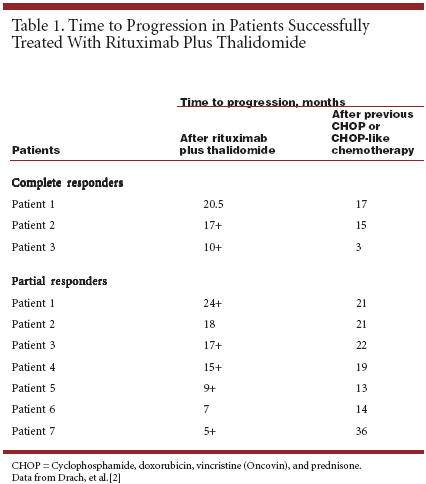
Eleven patients were evaluable: one with primary mantle cell lymphoma resistant to CHOP, seven enrolled at first relapse, and three enrolled at second or further relapse. The median time from diagnosis was 21 months (range, 6 to 53 months). No Unexpected Side Effects This treatment regimen was safe and well tolerated, and no unexpected side effects were encountered with the addition of thalidomide to rituximab therapy. Because of its antian giogenic effects, thalidomide can increase the risk for thromboembolic events, particularly when adminis- tered in combination with chemotherapy.[ 3] In this study using thali- domide in combination with rituximab, thromboembolic events were observed in two patients.[2] One patient discontinued therapy because of severe neutropenia associated with the administration of thalidomide. The objective response rate was 91%, with three complete and seven partial responses observed. Notably, one of the complete responses was in the patient resistant to CHOP chemotherapy. Remissions were durable (see Table 1).[2] The time to progression for patients with a complete response was improved relative to their previous chemotherapy regimens. Overall, these results suggest that rituximab plus thalidomide has a favorable toxicity profile and marked antitumor activity in patients with relapsed or CHOP-resistant mantle cell lymphoma. Further study in a prospectively defined, homogenous patient population is warranted.
References:
1. Richardson P, Hideshima T, Anderson K: Thalidomide: emerging role in cancer medicine. Annu Rev Med 53: 629-657, 2002.
2. Drach J, Kaufmann H, Puespoek A, et al: Marked anti-tumor activity of rituximab plus thalidomide in patients with relapsed/resistant mantle cell lymphoma (abstract 606). Blood 100:162a, 2002.
3. Clark TE, Edom N, Larson J, Lindsey LJ: Thalomid (thalidomide) capsules: a review of the first 18 months of spontaneous postmarketing adverse event surveillance, including off-label prescribing. Drug Saf 24:87-117, 2001.
Highlighting Insights From the Marginal Zone Lymphoma Workshop
Clinicians outline the significance of the MZL Workshop, where a gathering of international experts in the field discussed updates in the disease state.