Fludarabine/Cyclophosphamide/ Rituximab Combination Achieves High Molecular Remission Rate
This special supplement to Oncology NewsInternational includes updated results ofstudies with anti-CD20 therapy and othertargeted therapies in the treatment oflymphomas, chronic lymphocytic leukemia,and immune thrombocytopenic purpura. Theresults were presented at the American Societyof Hematology 44th Annual Meeting inPhiladelphia, December 6 to 10, 2002.
HOUSTON-As treatments forchronic lymphocytic leukemia (CLL)improve, the definitions of completeremission (CR) and partial remission(PR) have become increasingly refined.The National Cancer InstituteWorking Group (NCIWG) also recognizesother types of responses, includingnodular partial remission(nPR), defined as complete remissionexcept for residual lymphocyte aggregatesin bone marrow on biopsy. Inaddition, many CR patients haveclonal disease detectable on flow cytometryand molecular remissions,defined as negative by polymerasechain reaction (PCR) for the IgVhgene.Because of the indolent, relapsingnature of CLL, there is interest in developingtreatment regimens that havehigh molecular remission rates in thehope that achieving molecular remissionmay increase the duration of completeremission. Rituximab (Rituxan)has been shown to improve the molecularremission rate after chemotherapy.[1]FCR RegimenA combination regimen of fludarabine(Fludara) (25 mg/m2/day for 3days), cyclophosphamide (Cytoxan,Neosar, 250 mg/m2/day for 3 days),and rituximab (375 to 500 mg/m2 onday 1), the FCR regimen, was admin-istered to 135 previously untreatedpatients with CLL (ASH abstract771).[2] Michael Keating, MD, andfellow investigators at the Universityof Texas M.D. Anderson Cancer Center,Houston, then compared themolecular remission rate to the NCIWG response criteria and clinical response.
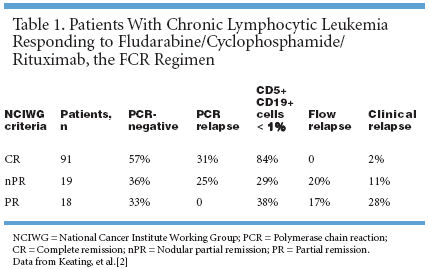
According to NCIWG criteria, responserates were: 67% CR, 19% nPR,and 18% PR. Of these, 9 respondershave relapsed clinically and 12 patientshave died: 2 with CR, 1 withnPR, 4 with PR, and 5 of the 7 nonresponders.The percentage of respondingpatients for each endpoint is summarizedin Table 1.[2]Insummary, FCR achieved highcomplete remission and molecularremission rates in patients with previouslyuntreated CLL. The study suggeststhat molecular remission maycorrelate with good clinical prognosis.
References:
1.
Wierda W, O’Brien S, Albitar M,et al: Combined fludarabine, cyclophosphamide,and rituximab achievesa high complete remission rate as initialtreatment for chronic lymphocyticleukemia (abstract 3210). Blood98:771a, 2001.
2.
Keating M, Manshouri T,O’Brien S, et al: A high proportion ofmolecular remission can be obtainedwith a fludarabine, cyclophosphamide,rituximab combination (FCR)in chronic lymphocytic leukemia(CLL) (abstract 771). Blood 100:205a,2002.
Oncology Peer Review On-The-Go: Minority Treatment Disparities and Clinical Trial Enrollment
July 6th 2020The first episode of CancerNetwork's podcast Oncology Peer Review On-The-Go explores disparities in cancer care treatment among minorities and the significance of a representative sample in clinical trials.