Adding Anti-CD20 Antibody to First-Line Chemotherapy Improves Response in Previously Untreated NHL
This special supplement to Oncology NewsInternational includes updated results ofstudies with anti-CD20 therapy and othertargeted therapies in the treatment oflymphomas, chronic lymphocytic leukemia,and immune thrombocytopenic purpura. Theresults were presented at the American Societyof Hematology 44th Annual Meeting inPhiladelphia, December 6 to 10, 2002.
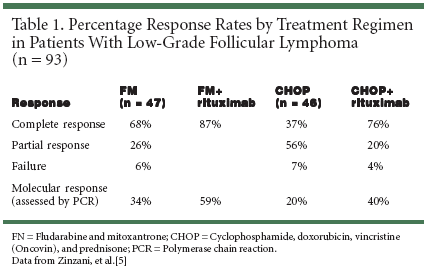
PHILADELPHIA-The combinationof fludarabine (Fludara) andmitoxantrone (Novantrone), the FMregimen, has demonstrated high responserates in newly diagnosed follicularlymphoma.[1] Previous studieswith this combination chemotherapyhave shown overall response ratesranging from 55% in intermediate/high-grade non-Hodgkin's lymphoma(NHL) to 89% in low-gradeNHL.[2,3]Adding rituximab (Rituxan), a Bcell-depleting, anti-CD20 antibody,may further increase response rateswhile adding little additional toxicityto the FN regimen. This hypothesiswas tested by Stephanie A. Gregory,MD, and colleagues at Rush-Presbyterian-St. Luke's Medical Center inChicago, Boston Baskin Cancer Groupin Memphis, and the CommunityHospital of Munster, Indiana.Dr. Gregory reported that the FNregimen followed by rituximabachieves high response rates in previouslyuntreated patients with lowgrade,advanced NHL (ASH poster1401).[4]Phase II StudyThis phase II study evaluated andreported on 41 patients with CD20+previously untreated NHL. Histologicdiagnosis showed that 11 patients(27%) had small lymphocytic lymphoma;18 (44%) had follicular smallcleaved lymphoma; 6 (15%) had fol-licular mixed lymphoma; 3 (7%) hadmaltoma; 2 (5%) had mantle cell lymphoma;and 1 (2%) had marginalzone lymphoma. Patients were treatedwith four to six cycles (28 days percycle) of fludarabine (25 mg/m2 ondays 1 to 3) and mitoxantrone (12mg/m2 on day 1) with no prophylacticantibiotic therapy.Patients who achieved a completeresponse after four or six cycles of theFN regimen received rituximab (375mg/m2/week for 4 weeks) 4 to 6 weeksafter cessation of chemotherapy. Allpatients had a Karnofsky performancestatus greater than or equal to 70.Overall, 33 patients (80%) had stageIV disease, 3 (8%) had stage III disease,and 5 (12%) had stage II disease.Clinical ResponseOf 31 evaluable patients, 30 (97%)achieved a clinical response, including14 (45%) complete responses and16 (52%) partial responses. These responseshave been durable, with 50%of patients who achieved a completeresponse remaining in remission formore than 24 months and 31% ofpatients who achieved a partial responseremaining in remission formore than 12 months.Serious adverse events reportedduring the study included grade 3 and4 neutropenia (n = 64 events in 163chemotherapy cycles), neutropenicfever (n = 7), dyspnea (n = 2), retinaldetachment (n = 1), and thrombusformation (n = 1). Three patients requireda 25% dose adjustment forprolonged neutropenia and two patientsdiscontinued because of neutropenia.Although prophylactic antimicrobialtherapy was not used,infections were rare, with only onecase of pneumonia.Comparison With CHOPAnother evaluation of the FN regimenfollowed by rituximab in treatmentof low-grade lymphoma [5] waspresented by Pier Luigi Zinzani, MD,on behalf of the Italian CooperativeStudy Group on Lymphoma, Instituteof Hematology and Medical Oncology,University of Bologna, Italy(ASH abstract 344). In this study, theefficacy and safety of the FM regimenfollowed by rituximab were comparedwith the efficacy and safety of cyclophosphamide(Cytoxan, Neosar),doxorubicin, vincristine (Oncovin),and prednisone (CHOP) followed byrituximab in patients with previouslyuntreated follicular lymphoma.Patients with a histologically confirmeddiagnosis of CD20+ follicularlymphoma (according to RevisedEuropean-American Lymphoma[REAL] classification standards) anda positive diagnosis of bcl-2/IgHrearrangement by polymerase chainreaction were randomized to receivesix cycles of either fludarabine (25mg/m2/day IV on days 1 to 3) andmitoxantrone (10 mg/m2 IV on day1) or CHOP (cyclophosphamide 750mg/m2 IV on day 1, doxorubicin 50mg/m2 IV on day 1, vincristine 1.4mg/m2 IV on day 1, and prednisone100 mg/day on days 1-5). Other inclusioncriteria included age 18 to 70years, stage II to IV disease, and anEastern Cooperative Oncology Groupperformance status less than or equalto 2.Patients who achieved partial orcomplete responses but were still positivein bone marrow and/or peripheralblood (as assessed by polymerasechain reaction at 4 and 6 weeks afterchemotherapy) were eligible to receivefour cycles of 375 mg/m2 IVrituximab per week to determine ifrituximab treatment could eliminateminimal residual disease.Of 159 randomized patients, 93were evaluable for response. Rituximabimproved the clinical and molecularresponse rates for both treatmentregimens. The results arepresented in Table 1.[5]These results suggest that the FMregimen may be more effective thanCHOP for first-line treatment of patientswith follicular lymphoma, andthat addition of rituximab to eitherchemotherapy regimen improves response,in some cases dramatically.Further evaluation will be necessaryto confirm these results. Overall, thesestudies show that the combination ofFM followed by rituximab is a verypromising regimen that leads to highresponse rates and durable remissionsin previously untreated patients withlow-grade or follicular NHL.
References:
1.
Armitage JO. The role of mitoxantronein non-Hodgkin’s lymphoma.Oncology 16:490-502, 507-8; discussion511-2, 514, 2002.
2.
Gregory SA, Vose J, ModianoM, et al: Mitoxantrone and fludarabinein the treatment of patients withnon-Hodgkin’s lymphoma failingprimary therapy with a doxorubicinor mitoxantrone-containing regimen.Leuk Lymphoma 40:315-324, 2001.
3.
Zinzani PL, Magagnoli M, BendandiM, et al: Efficacy of fludarabineand mitoxantrone combination regimenin untreated indolent non-Hodgkin’s lymphomas. Ann Oncol11:363-365, 2000.
4.
Gregory SA, Venugopal V, AdlerSS, et al: Combined fludarabine, mitoxantrone,and rituximab achieves ahigh response as initial treatment foradvanced low grade non-Hodgkin’slymphoma (LGNHL). Poster 1401presented at: American Society ofHematology 44th Annual Meeting,Philadelphia, December 6-10, 2002.
5.
Zinzani PL. A multicenter randomizedtrial of fludarabine and mitoxantrone(FM) plus rituximab versusCHOP plus rituximab as first-linetreatment in patients with follicularlymphoma (FL) (abstract 344). Blood100:93a, 2002.
Oncology Peer Review On-The-Go: Minority Treatment Disparities and Clinical Trial Enrollment
July 6th 2020The first episode of CancerNetwork's podcast Oncology Peer Review On-The-Go explores disparities in cancer care treatment among minorities and the significance of a representative sample in clinical trials.