Multiple Myeloma: A Clinical Overview
Multiple myeloma (MM) is a malignant, progressive plasma cell tumor characterized by overproduction of monoclonal immunoglobulins, osteolytic bone lesions, renal disease, and immunodeficiency.[1] Before the 1980s, patients with MM experienced a slow, progressive decline in quality of life until death approximately 2 years after diagnosis.
Multiple myeloma (MM) is the second most common hematologic malignancy in the United States, affecting slightly more men than women and twice as many African Americans as Caucasians. Older age is the primary risk factor for MM, but obesity also increases risk. MM is incurable, but treatment advances in the past decade have more than doubled the duration of survival. MM is a progressive plasma cell tumor in which an initially stable clone becomes malignant via a multistep process. Causative factors implicated in this process include radiation, environmental toxins, chronic antigen stimulation, and genetics. The malignant plasma cells interact with other hematopoietic and stromal cells within the bone marrow microenvironment to disrupt homeostasis among cells and within the extracellular matrix. These tumor-host interactions lead to MM cell proliferation and migration, angiogenesis, osteolysis, immunodeficiency, and anemia. As a result, patients often present with osteolytic bone lesions, recurrent infections, renal insufficiency, and fatigue. The Durie-Salmon and International Staging Systems are used to stage MM, with the latter providing prognostic information based on readily available laboratory data. However, a number of cytogenetic markers are emerging as prognostic indicators, introducing the possibility of more refined disease staging systems and tailored treatment strategies based on genetic profiles.
Introduction
Multiple myeloma (MM) is a malignant, progressive plasma cell tumor characterized by overproduction of monoclonal immunoglobulins, osteolytic bone lesions, renal disease, and immunodeficiency.[1] Before the 1980s, patients with MM experienced a slow, progressive decline in quality of life until death approximately 2 years after diagnosis,[2] but the advent of high-dose alkylating agents increased the probability of remission and prolonged overall and event-free survival. During the past decade, important advances have been made in understanding the cellular and molecular mechanisms of MM, leading to the development of even more effective treatment strategies,[3,4] including stem cell transplantation, the immunomodulators thalidomide and lenalidomide, and the first-generation proteasome inhibitor bortezomib.
Currently, MM is an active field of research for novel pharmacotherapies, with a number of agents in phase II or III of clinical development. This supplement describes several therapeutic advances in the context of the underlying molecular mechanisms. The objective of this article is to put these advances into the current clinical context by providing an overview of the epidemiology, etiology, and clinical features of MM, along with the prognosis for patients with this disease.
Incidence and Mortality
According to estimates from the American Cancer Society, 20,180 new cases of MM were diagnosed in the United States and an estimated 10,650 Americans died of MM in 2010.[5] MM is the second most common hematologic malignancy after non-Hodgkin lymphoma and represented nearly 15% of new hematologic malignancies diagnosed in 2010.[5] Globally, the incidence of MM ranges from around 1 per 100,000 in China to around 4 per 100,000 in most developing countries.[1]
Although MM is currently an incurable disease, treatment advances during the past decade have translated into a decrease in the mortality rate.[6] Before the 1980s, patients usually died within 2 years of receiving a diagnosis of MM. In contrast, since the year 2000 median overall survival with treatment has ranged from 4.4 to 7.1 years.[2]
Demographics
The incidence of MM is slightly higher in men than in women, and about twice as high in African Americans as in Caucasians.[1,7] Asians appear to have a lower risk of developing MM than Caucasians or persons of African descent.[8] Persons of African descent are also more likely than Caucasians to develop the MM precursor, monoclonal gammopathy of undetermined significance (MGUS).[8-10] Actually, the difference in the incidence of MM between Caucasians and African Americans appears to be the result of a higher rate of MGUS among African Americans, rather than a higher rate of conversion from MGUS to MM.[9] The racial discrepancy in MGUS or MM incidence may be partially related to socioeconomic factors,[11] although no association has been proved between the risk of MM and the socioeconomic factors of family income, education level, occupation, dwelling size, or crowding in the home,[12] or between MGUS and education level or household income.[10] In clinical trials, the age-standardized rate of death is similar across all ethnicities for patients with MM[13]; similarly, the current decline in MM mortality is seen in all ethnic groups, although it has affected Native Americans the least.[6]
MM is generally a disease of older persons.[7] The median age at diagnosis is between 61 and 68 years,[1,2,4,14] with approximately 2% of patients < 40 years.[1,14]
There is growing evidence of a significant association between being overweight or obese and the development of MM[15,16] and MGUS.[10] Overweight and obesity are also predictors of MM-associated mortality.[15] The relationship between body mass index and MM incidence is similar across sexes and ethnicities in both the United States and Europe.[15] The mechanism for this relationship is not clear, but it may involve the proinflammatory cytokines interleukin-6 or insulin-like growth factor, which may be elevated in obesity[17,18] and which mediate proliferation of myeloma cells.[18,19] There have been some indications of a familial predisposition to the development of MM, and studies are ongoing to evaluate families in which there are many individuals with myeloma.[20]
Etiology
FIGURE 1
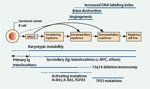
The Pathogenic Progression of Multiple Myeloma
MM is characterized by the pernicious transformation of a plasma cell precursor into a plasma cell myeloma, and it is actually the final malignant phase of a disease process that begins with the relatively benign MGUS (Figure 1).[7] In the premalignant phase of MGUS, patients may be asymptomatic and only have elevated levels of idiotypic monoclonal (M) immunoglobulin (Ig); this phase can remain stable for many years.[7] “Smoldering” myeloma describes the transition phase between MGUS and frank MM, when patients have an intramedullary tumor cell content of >10% but no osteolytic lesions or other complications of MM.[2,7,21] Each year, about 1% of patients with MGUS progress to symptomatic MM or another form of plasma cell neoplasia.[22] The diagnosis of MM at any of these stages can be difficult; therefore, the distinctions between the phases and subtypes of myeloma have been defined by the International Myeloma Working Group (Table 1).[21]
TABLE 1

International Myeloma Working Group Criteria for Monoclonal Gammopathies and MM[21]
Progression to MM probably reflects a combination of genetic changes in the malignant plasma cell, alterations in the bone marrow microenvironment (supporting tumor growth), and failure of the immune system to control the disease.[7] The induction of MM is probably a multistep process involving formation of the initial plasma cell clone and then conversion of the stable clone into a progressive malignant tumor. Factors implicated in these processes include radiation exposure, environmental exposure, chronic antigen stimulation, and genetics.[7]
Radiation exposure
Data on the potential impact of ionizing radiation exposure on the development of MM are inconsistent.[7] The risk of developing MM,[23] but not MGUS,[24] was increased in Hiroshima/Nagasaki atomic bomb survivors-but only 20 years after radiation exposure-and the risk was dose-dependent.[23] Although the overall risk of MGUS was not increased, atomic bomb survivors tended to develop MGUS earlier than age-matched controls and showed more rapid conversion from MGUS to MM, although these findings were not statistically significant.[25] Personnel from the United Kingdom who were present at atmospheric nuclear tests and who were exposed to high-dose, short-duration radiation did not show an increase in MM incidence.[26] However, these data have been criticized for omitting some MM cases from the cohort and thereby underestimating the true MM incidence.[27] On the other hand, long-duration exposure to lower doses of radiation may be associated with a slight increase in the incidence of MM, such as that seen in individuals working in the nuclear industry.[28,29] In these individuals, older age at the time of radiation exposure, rather than lifetime cumulative radiation dose, appeared to be the more significant risk factor for MM development.[28] Radiotherapy treatments for pelvic cancers, however, do not increase the risk of MM development.[30]
Environmental exposure
An increased incidence of MM has been demonstrated in particular occupations, including farming, painting, metalworking, rubber manufacturing, woodworking, leather and textile processing, and petroleum production, although these relationships have not been consistently demonstrated across different studies.[7] Farming and agriculture have the strongest relationship to MM development[7,31]; these relationships may reflect exposure to pesticides or zoonotic infectious agents.[31]
Chronic antigen stimulation
It is possible that chronic antigen stimulation, with its associated lymphocyte activation, also plays a role in MM development.[7] A number of studies have shown a higher than expected incidence of MM among patients with rheumatoid arthritis (RA).[32-34] However, factors other than chronic antigen stimulation may play a role, such as a shared predisposition for the development of RA and MM (evidenced by simultaneous development of MGUS and RA in some patient series[35] and by a high rate of RA among first-degree relatives of patients with MM[36]). Another factor may be the effects of medications, such as corticosteroids, which are associated with an increased risk of MM, at least in women.[37]
Certain viral infections have also been implicated, albeit with variable findings and quality of supporting data. These include hepatitis C virus,[38] hepatitis B virus,[39] and human immunodeficiency virus.[40,41]
Genetics
The familial and racial patterns of MM suggest genetic links.[7] Almost 50% of all patients with MM have a first-degree relative who has had cancer,[14] and the risk for both MM and MGUS is twice as high in persons with a first-degree relative who has had cancer.[42,43]
Features of the Disease
Immature B cells are characterized by a certain degree of inherent genetic instability because of the many recombination events necessary for them to mature into plasma cells.[2] Primary genetic translocations in MM frequently occur in the Ig heavy-chain loci[2]; the incidence of Ig heavy-chain translocations increases with advancing stage of tumorigenesis.[7] Secondary translocations occur later in the disease course and include complex abnormalities of c-MYC; activation of N-RAS, K-RAS, and FGFR3 mutations; inactivation, mutations, or deletions of TP53, RB1, and PTEN; and inactivation of cyclin-dependent kinase (CDK) inhibitors CDKN2A and CDKN2C (Figure 1).[1,7] The types of translocations identify prognostic subgroups of patients, and ongoing research in this area may help to tailor treatment strategies to individual patients based on their cytogenetic profiles.[1]
FIGURE 2
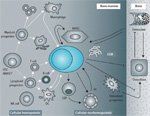
Interactions of MM Cells With the Cellular Hemopoietic and Nonhemopoietic Bone Marrow Compartments
Once formed, malignant plasma cells interact with hematopoietic and stromal cells within the bone marrow microenvironment to disrupt homeostasis between cells and within the extracellular matrix (Figure 2).[1] These interactions cause increased bone resorption (osteolysis, osteoclastogenesis, and inhibition of osteogenesis) and humoral and cellular immunodeficiency.[1,7] In addition, bone marrow stromal cells induce direct (via cell-cell interactions) and indirect (via secretion of soluble factors) signaling cascades that support MM cell proliferation, survival, migration, and drug resistance, as well as tumor angiogenesis.[1,7]
As a result, most patients with MM present with bone pain or fractures of unknown etiology, renal failure, or recurrent infections.[2] Anemia and fatigue are also common.[14] A confirmatory diagnosis of MM is made after identification and quantification of Igs, M-protein, and Ig light chains (? and λ) in blood or urine by electrophoresis and immunofixation[4,21]; radiographic evidence of typical bone lesions; and confirmation of the presence of plasma cells in bone marrow aspirates.[2,4]
Disease Staging and Prognostic Indicators
TABLE 2
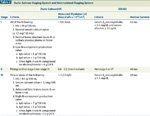
Durie-Salmon Staging System and International Staging System
The Durie-Salmon system, which is based on variables correlated with tumor mass (Table 2), has been used since the 1970s to stage disease severity in MM.[44] More recently, the International Staging System (ISS) was developed; this system is based on statistical modeling of prognostic indicators for survival (see Table 2).[45] The ISS has a number of advantages over the Durie-Salmon system. First, it is easier to use since it is based on readily available laboratory parameters.[4] Second, there is a more even distribution of patients between stages[45] and better discrimination between stages.[7] Third, the ISS has been validated across different geographic regions, age groups, and treatment types.[45]
In addition to disease stage, other factors have been identified as predictive of poor outcome in patients with MM, including absence of the IgA isotype, creatinine level ≥ 2 mg/dL, and low platelet count.[46] Among patients receiving conventional therapy, the factors of older age, poor performance status, presence of serum M-protein, low hemoglobin levels, and bone marrow plasma cell infiltration (≥ 33%) also independently predict worse outcome.[46] Because MM is a tumor with a slow rate of proliferation, the plasma cell labeling index (a reflection of the rate of tumor cell proliferation) is a better prognostic indicator than the tumor cell content of bone marrow.[7]
Currently, a number of cytogenetic markers are emerging as prognostic indicators in patients with MM (Table 3).[1] More refined prognostic models or disease staging systems may be developed that incorporate these markers.
Refining Our Understanding of Myeloma
TABLE 3
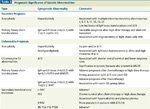
Prognostic Significance of Genetic Abnormalities
Ongoing translational research is likely to drive the development of novel therapies for MM through a number of parallel approaches. First, there is significant interest in determining the underlying genetics and epigenetics of myeloma genesis, including identification of novel MM oncogenes or suppressor genes that may serve as potential therapeutic targets.[47] Recently, Chapman and colleagues conducted a preliminary genomic analysis of samples from 38 patients with MM by performing whole-genome sequencing or whole-exome sequencing. Tumor-specific mutations were identified by comparing each tumor to a corresponding normal sample. A striking finding of the study was the discovery of mutations in genes involved in RNA processing, protein translation, and the unfolded protein response in nearly half of the patients. The authors’ analysis revealed potentially broader roles for mechanisms previously suspected to have a role in the biology of MM (eg, nuclear factor kappa B [NFκB] activation, histone methyltransferase [HMT] dysfunction), and identified new candidate mechanisms of transformation, including mutations in the RNA exonuclease DIS3, putative mRNA stability factor FAM46C, and other genes involved in protein translation and homeostasis.[48] Additional efforts are underway to improve the classification and development of personalized novel agents for the treatment of MM, based in part on the profiling of patients over time, since studies have shown continued evolution of genetic changes with the progression of MM.[47] Key developments are also in the works for next-generation novel therapies targeting MM cells in the bone marrow microenvironment; rationally based combination therapies; and immune therapies, including monoclonal antibodies and MM-specific peptide vaccines.[47]
Conclusions
Advances in our knowledge of the molecular, cellular, and genetic bases of MM are leading to improvements in diagnosis, disease staging, and treatment-and these improvements are reflected in reduced mortality rates and lengthened survival durations. It is likely that future treatment approaches will be specifically tailored to individual patients based on their demographic, clinical, and genetic profiles. While the ultimate goal of a cure for MM remains elusive, these developments may help to produce long-lasting remissions with optimal quality of life for a large proportion of MM patients.
Acknowledgments: The author would like to thank Catherine Rees, PhD, and Brian E. Szente, PhD, of Fishawack Communications for their assistance with manuscript development. This editorial support was funded by Onyx Pharmaceuticals.
References:
REFERENCES
1. Raab MS, Podar K, Breitkreutz I, et al. Multiple myeloma. Lancet. 2009;374:324-39.
2. Sirohi B, Powles R. Multiple myeloma. Lancet. 2004;363:875-87.
3. Laubach JP, Richardson PG, Anderson KC. The evolution and impact of therapy in multiple myeloma. Med Oncol. 2010;27(suppl 1):S1-6.
4. Anderson KC, Alsina M, Bensinger W, et al. NCCN clinical practice guidelines in oncology: multiple myeloma. J Natl Compr Canc Netw. 2009;7:908-42.
5. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 60:277-300.
6. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544-73.
7. Bergsagel PL. Epidemiology, etiology, and molecular pathogenesis. In: Anderson KC, editor. Multiple myeloma. London: Remedica; 2003. p. 17-37.
8. Landgren O, Weiss BM. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic/racial groups: support for genetic factors in pathogenesis. Leukemia. 2009;23:1691-7.
9. Landgren O, Gridley G, Turesson I, et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood. 2006;107:904-6.
10. Landgren O, Rajkumar SV, Pfeiffer RM, et al. Obesity is associated with an increased risk of monoclonal gammopathy of undetermined significance among black and white women. Blood. 2010;116:1056-9.
11. Baris D, Brown LM, Silverman DT, et al. Socioeconomic status and multiple myeloma among US blacks and whites. Am J Public Health. 2000;90:1277-81.
12. Johnston JM, Grufferman S, Bourguet CC, et al. Socioeconomic status and risk of multiple myeloma. J Epidemiol Community Health. 1985;39:175-8.
13. Albain KS, Unger JM, Crowley JJ, et al. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984-92.
14. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21-33.
15. Larsson SC, Wolk A. Body mass index and risk of multiple myeloma: a meta-analysis. Int J Cancer. 2007;121:2512-6.
16. Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569-78.
17. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579-91.
18. Lauta VM. A review of the cytokine network in multiple myeloma: diagnostic, prognostic, and therapeutic implications. Cancer. 2003;97:2440-52.
19. Georgii-Hemming P, Wiklund HJ, Ljunggren O, Nilsson K. Insulin-like growth factor I is a growth and survival factor in human multiple myeloma cell lines. Blood. 1996;88:2250-8.
20. Lynch HT, Ferrara K, Barlogie B, et al. Familial myeloma. N Engl J Med. 2008;359:152-7.
21. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749-57.
22. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564-9.
23. Ichimaru M, Ishimaru T, Mikami M, Matsunaga M. Multiple myeloma among atomic bomb survivors in Hiroshima and Nagasaki, 1950-76: relationship to radiation dose absorbed by marrow. J Natl Cancer Inst. 1982;69:323-8.
24. Neriishi K, Yoshimoto Y, Carter RL, et al. Monoclonal gammopathy in atomic bomb survivors. Radiat Res. 1993;133:351-9.
25. Neriishi K, Nakashima E, Suzuki G. Monoclonal gammopathy of undetermined significance in atomic bomb survivors: incidence and transformation to multiple myeloma. Br J Haematol. 2003;121:405-10.
26. Muirhead CR, Bingham D, Haylock RG, et al. Follow up of mortality and incidence of cancer 1952-98 in men from the UK who participated in the UK's atmospheric nuclear weapon tests and experimental programmes. Occup Environ Med. 2003;60:165-72.
27. Roff SR. Under-ascertainment of multiple myeloma among participants in UK atmospheric atomic and nuclear weapons tests. Occup Environ Med. 2003;60:e18.
28. Wing S, Richardson D, Wolf S, et al. A case control study of multiple myeloma at four nuclear facilities. Ann Epidemiol. 2000;10:144-53.
29. Yiin JH, Anderson JL, Daniels RD, et al. A nested case-control study of multiple myeloma risk and uranium exposure among workers at the Oak Ridge Gaseous Diffusion Plant. Radiat Res. 2009;171:637-45.
30. Wright JD, St Clair CM, Deutsch I, et al. Pelvic radiotherapy and the risk of secondary leukemia and multiple myeloma. Cancer. 2010;116:2486-92.
31. Mester B, Nieters A, Deeg E, et al. Occupation and malignant lymphoma: a population based case control study in Germany. Occup Environ Med. 2006;63:17-26.
32. Eriksson M. Rheumatoid arthritis as a risk factor for multiple myeloma: a case-control study. Eur J Cancer. 1993;29A:259-63.
33. Hakulinen T, Isomaki H, Knekt P. Rheumatoid arthritis and cancer studies based on linking nationwide registries in Finland. Am J Med. 1985;78:29-32.
34. Katusic S, Beard CM, Kurland LT, et al. Occurrence of malignant neoplasms in the Rochester, Minnesota, rheumatoid arthritis cohort. Am J Med. 1985;78:50-5.
35. Jorgensen C, Guerin B, Ferrazzi V, et al. Arthritis associated with monoclonal gammapathy: clinical characteristics. Br J Rheumatol. 1996;35:241-3.
36. Linet MS, McLaughlin JK, Harlow SD, Fraumeni JF. Family history of autoimmune disorders and cancer in multiple myeloma. Int J Epidemiol. 1988;17:512-3.
37. Landgren O, Zhang Y, Zahm SH, et al. Risk of multiple myeloma following medication use and medical conditions: a case-control study in Connecticut women. Cancer Epidemiol Biomarkers Prev. 2006;15:2342-7.
38. Montella M, Crispo A, Frigeri F, et al. HCV and tumors correlated with immune system: a case-control study in an area of hyperendemicity. Leuk Res. 2001;25:775-81.
39. Franceschi S, Lise M, Trepo C, et al. Infection with hepatitis B and C viruses and risk of lymphoid malignancies in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Epidemiol Biomarkers Prev. 2011;20:208-14.
40. Amara S, Dezube BJ, Cooley TP, et al. HIV-associated monoclonal gammopathy: a retrospective analysis of 25 patients. Clin Infect Dis. 2006;43:1198-205.
41. Pulik M, Genet P, Jary L, et al. Acute myeloid leukemias, multiple myelomas, and chronic leukemias in the setting of HIV infection. AIDS Patient Care STDS. 1998;12:913-9.
42. Kristinsson SY, Bjorkholm M, Goldin LR, et al. Patterns of hematologic malignancies and solid tumors among 37,838 first-degree relatives of 13,896 patients with multiple myeloma in Sweden. Int J Cancer. 2009;125:2147-50.
43. Vachon CM, Kyle RA, Therneau TM, et al. Increased risk of monoclonal gammopathy in first-degree relatives of patients with multiple myeloma or monoclonal gammopathy of undetermined significance. Blood. 2009;114:785-90.
44. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842-54.
45. Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412-20.
46. Ludwig H, Durie BG, Bolejack V, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group. Blood. 2008;111:4039-47.
47. Anderson KC. Oncogenomics to target myeloma in the bone marrow microenvironment. Clin Cancer Res. 2011;17:1225-33.
48. Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471:467-72.
Navigating AE Management for Cellular Therapy Across Hematologic Cancers
A panel of clinical pharmacists discussed strategies for mitigating toxicities across different multiple myeloma, lymphoma, and leukemia populations.