Treatment-Related Adverse Events in Patients With Relapsed/Refractory Multiple Myeloma
The rational development of novel targeted therapies is expanding treatment options for patients with relapsed/refractory (R/R) multiple myeloma (MM). The first-in-class proteasome inhibitor (PI) bortezomib (Velcade), the immunomodulatory agents thalidomide (Thalomid) and lenalidomide (Revlimid), and liposomal doxorubicin are currently the major approved therapeutic agents in this setting.[1]
In the past decade we have seen four new agents approved by the US Food and Drug Administration for treatment of multiple myeloma: the proteasome inhibitor (PI) bortezomib (Velcade), the immunomodulatory agents lenalidomide (Revlimid) and thalidomide (Thalomid), and liposomal doxorubicin. These are commonly used in the treatment of relapsed/refractory (R/R) multiple myeloma (MM), but there is no universally accepted standard treatment. Salvage therapy must be tailored according to an individual patient’s clinical profile, with the risks and potential effects of treatment-related adverse events being major determinants of the choice of therapy. Two novel agents in phase II studies to investigate their potential for the treatment of R/R MM are carfilzomib, a selective, irreversible next-generation PI, and pomalidomide, a next-generation thalidomide analog. This review will discuss the side-effect profiles of the currently approved immunomodulatory agents and bortezomib, as well as those of the newer agents, carfilzomib and pomalidomide.
Introduction
TABLE 1
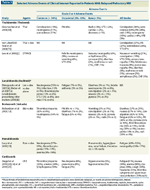
Selected Adverse Events of Clinical Interest Reported in Patients With Relapsed/Refractory MM
The rational development of novel targeted therapies is expanding treatment options for patients with relapsed/refractory (R/R) multiple myeloma (MM). The first-in-class proteasome inhibitor (PI) bortezomib (Velcade), the immunomodulatory agents thalidomide (Thalomid) and lenalidomide (Revlimid), and liposomal doxorubicin are currently the major approved therapeutic agents in this setting.[1] Other novel agents, such as the next-generation thalidomide analog pomalidomide and the more selective next-generation PI carfilzomib, are under investigation for the treatment of R/R MM.
The newer agents for the treatment of R/R MM have distinct toxicity profiles (Table 1).[2] The effective management of the adverse events (AEs) associated with these agents is crucial to ensure that patients receive the most effective dosing regimen while maintaining an acceptable quality of life.
Important Treatment-Related Adverse Events
Hematologic adverse events
Thalidomide and lenalidomide. Myelosuppression is an AE commonly associated with lenalidomide therapy, mainly manifesting as neutropenia and thrombocytopenia.[3] In two phase III studies in patients with R/R MM, grade 3/4 neutropenia occurred in approximately 30% to 40% of patients treated with lenalidomide + dexamethasone, compared with only 2.3% to 4.5% of patients treated with dexamethasone alone, although grade 3/4 febrile neutropenia occurred in less than 4% of patients.[4,5] In contrast, treatment with thalidomide is associated with only mild neutropenia in 3% to 15% of patients.[3,6] Thrombocytopenia was the second most common hematologic toxicity in patients treated with lenalidomide + dexamethasone, with grade 3/4 thrombocytopenia occurring in 11% to 15% of patients.[4,5]
In one of the earliest dose-finding studies of thalidomide for the treatment of refractory MM, grade 3 or 4 thrombocytopenia or anemia occurred in only 3 of 258 patients (1.2%).[7] In most of the patients who had no response, pretreatment anemia or thrombocytopenia did not worsen, although significant increases in hemoglobin levels occurred in responding patients.[7] Anemia has also been observed following treatment with lenalidomide. In pivotal trials involving > 700 patients, the incidence of anemia was 31.4% with lenalidomide + dexamethasone vs 23.7% in the dexamethasone-only group.[8] In the same studies, the incidence of grade 3/4 anemia was 10.8% in the lenalidomide + dexamethasone groups compared with 6.0% in the dexamethasone-only groups.[4,5]
Thalidomide-based regimens have been reported to impair CD34+ stem-cell yields in patients with newly diagnosed MM.[9,10] A more prominent trend toward lower CD34+ stem-cell yields has been observed in newly diagnosed patients treated with lenalidomide + dexamethasone, so it is recommended that peripheral blood stem cells be harvested before prolonged exposure to lenalidomide.[1,11] Duration of treatment with lenalidomide has also been cited as a reason for decreased stem-cell yields.[12]
Bortezomib. Thrombocytopenia is a prominent hematologic toxicity associated with bortezomib.[3] Grade 3/4 thrombocytopenia was reported in 30% of patients with R/R MM treated with bortezomib in the Assessment of Proteasome Inhibition for Extending Remissions (APEX) study.[13] Platelet levels typically decrease during each cycle of bortezomib treatment, returning toward baseline between cycles without evidence of cumulative thrombocytopenia.[13,14] Predicted time for recovery of platelet counts from nadir to baseline is less than 7 days with bortezomib, whereas other cytotoxic agents typically require 3 to 4 weeks for resolution.[14] Interestingly, a parallel phenomenon observed in murine models has been traced to a bortezomib-induced dose-dependent inhibition of proplatelet formation by megakaryocytes.[15]
Neutropenia associated with bortezomib follows a cyclical pattern, with nadirs occurring following the last dose of each cycle and typically recovering before initiation of the next cycle. In one phase II study of bortezomib in patients with R/R MM, neutrophil counts decreased during the bortezomib dosing period (days 1 to 11) and returned to baseline levels during the 10-day rest period at the end of each treatment cycle. This cyclical pattern of neutrophil decreases and recovery remained consistent over multiple cycles of twice-weekly dosing, with no evidence of cumulative neutropenia. Overall, neutropenia occurred in 19% of patients and was grade 3 in 11% to 12% of patients, with ≥ grade 4 in 2% to 3% of patients.[13,16] Neutropenia was reported as a serious event in < 1% of patients, and < 1% of patients discontinued treatment because of neutropenia. In the same study, the incidence of febrile neutropenia with bortezomib was < 1%.
Anemia was observed in studies evaluating the administration of bortezomib or dexamethasone in patients with relapsed MM, with a slightly increased incidence of anemia in patients treated with bortezomib compared with those treated with dexamethasone (26% vs 22%, respectively).[13]
Regarding CD34+ stem-cell toxicity, a study evaluating the impact of two cycles of bortezomib therapy prior to stem-cell collection showed no adverse effects on stem-cell yields in patients with peripheral blood mononuclear cells mobilized in response to granulocyte colony-stimulating factor.[17]
Pomalidomide. Grade 3/4 neutropenia has been observed in 37% to 55% of patients treated with pomalidomide (2 mg or 4 mg daily) plus low-dose dexamethasone in one study in patients with R/R MM.[18] In the same study, the incidence of grade 3/4 thrombocytopenia was 11% to 13%, while the incidence of grade 3/4 anemia was 9% to 16% across two dose cohorts. Neutropenia and anemia have also been noted as the most common grade 3/4 AEs in a second study evaluating the same combination regimen.[19] In that study, thrombocytopenia was also reported as a serious AE related to pomalidomide.[19] There are no data currently available on stem-cell toxicity related to treatment with pomalidomide.
Carfilzomib. The overall incidence of neutropenia in phase II trials of carfilzomib has been in the range of 17% to 31%, while grade 3/4 neutropenia was observed in 5% to 8% of patients.[20,21] Cyclic thrombocytopenia has also been observed in clinical trials with carfilzomib.[22,23] The overall incidence has ranged from 27% to 38%, and the incidence of grade 3/4 thrombocytopenia has ranged from 9% to 27%.[20,21] However, as with bortezomib, the clinical pattern of thrombocytopenia suggests that the underlying pathophysiology does not involve megakaryocytic or stem-cell injury.[14]
Treatment-emergent anemia was observed in 40% to 44% of patients in the major phase II trials of carfilzomib in patients with relapsed or refractory MM. These events were grade 3/4 in 5% to 20% of patients, although no patients discontinued treatment because of anemia.[20,21]
In a phase I/II study of the combination of carfilzomib, lenalidomide, and low-dose dexamethasone (CRd) for the treatment of newly diagnosed MM, all 14 patients underwent successful stem-cell harvest. The yields did not appear to be adversely impacted by the CRd regimen and produced a median of 6.15 × 106 CD34+ cells/kg (range, 4.1 to 8.5).[24].
TABLE 2
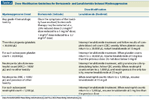
Dose-Modification Guidelines for Bortezomib- and Lenalidomide-Induced Myelosuppression
Management. Initial episodes of thrombocytopenia in the setting of bortezomib therapy are dealt with by withholding the next dose of bortezomib (Table 2).[25] Given the rapid reversibility of bortezomib-induced thrombocytopenia, it has been suggested that some patients with thrombocytopenia during treatment with bortezomib can continue therapy without dose interruptions.[14] Neutrophil counts should be monitored before giving each dose of bortezomib. Patients experiencing severe or prolonged episodes of thrombocytopenia or neutropenia may require a change in the dose and schedule of bortezomib, including dose interruptions.[6,25] Guidelines for the management of hematologic toxicity associated with lenalidomide are detailed in Table 2.
Anemia is commonly associated with MM itself and will often improve with treatment response.[6] Erythropoiesis-stimulating agents (ESAs) should be considered when, despite response to therapy, there has not been an increase in hemoglobin concentration.[6] However, ESAs may increase the risk of thromboembolism associated with immunomodulatory agents.
Neuropathy
Thalidomide and lenalidomide. Peripheral sensory neuropathy (PN) is frequently reported with thalidomide therapy.[6,26] The overall incidence of thalidomide-induced PN varies widely among studies (25% to 83%), with approximately 15% of patients needing to interrupt treatment.[26] Thalidomide-induced PN is typically characterized by stinging sensations or numbness in the extremities. It usually occurs after prolonged exposure and may be permanent.[26,27] Symptoms may occur even after thalidomide treatment has been stopped and may resolve slowly or not at all. Motor neuropathy, including trembling, may occur with thalidomide treatment. Deep vibratory sensitivity and proprioceptive changes may occur later in the course of treatment, resulting in progressive ataxia, difficulty in walking, and trembling when still.[26] Sinus bradycardia may occur as a result of autonomic neuropathy in MM patients receiving thalidomide and can be severe enough to cause syncope.[28]
The incidence of PN, however, is relatively low with lenalidomide,[26] suggesting that PN is not necessarily a class effect of immunomodulatory agents. In phase II trials of lenalidomide in patients with R/R MM, PN occurred in up to 10% of patients.[29,30] The incidence of grade 3/4 PN was similar in phase III trials, occurring in < 10% of patients with R/R MM receiving lenalidomide + dexamethasone.[4,5]
Bortezomib. PN is frequently reported with bortezomib therapy.[6,26] In a pooled analysis of 256 patients with R/R MM enrolled in the phase II SUMMIT and CREST trials of bortezomib, treatment-emergent PN occurred in 35% of patients overall, including 13% with grade 3 PN and 0.4% with grade 4 PN.[31] Dose reductions were necessary in 12% of patients, and 5% of patients discontinued therapy because of PN.[31] The clinical profile of bortezomib-induced PN includes neuropathic pain, mainly in the fingertips and toes, which may severely affect normal daily activities.[26,27] The risk of PN reaches a plateau by cycle 5, suggesting a dose threshold rather than a cumulative dose effect.[26,27] It improves or resolves in most patients at a median of 3 months after treatment is discontinued, but it has been reported that resolution can take as long as 2 years after treatment discontinuation.[27] Recent reports have indicated reduction in neuropathy with once-weekly dosing schedules and with subcutaneous administration.[32-34] Grade 1–3 bortezomib-induced motor neuropathy (involving distal weakness in the lower limbs) affects approximately 10% of patients.[26] Isolated cases of life-threatening grade 4 motor neurotoxicity have also been reported.[35]
The true incidence of bortezomib-induced autonomic neuropathy is not known. The fact that both constipation and diarrhea are associated with bortezomib in clinical trials may be relevant.[36] In a phase II study in patients with metastatic neuroendocrine tumors receiving a higher dose of bortezomib than the currently approved dose for MM, 6 of 10 patients with PN also had symptoms such as grade 2/3 dizziness, orthostatic hypotension, syncope, ileus, and abdominal cramps.[37] Pooled data from the phase II SUMMIT and CREST studies of bortezomib in patients with R/R MM revealed orthostatic hypotension in 12% of bortezomib-treated patients (grade 3 in 4% of patients).[26]
Two cases of grade 4 suspected autonomic neuropathy in patients with R/R MM receiving bortezomib included reports of abdominal numbness, distension, and urinary retention in one patient and persistent diarrhea, despite antibiotics and antimotility medication, in another patient.[35] Two additional patients with PN during bortezomib treatment were reported to have developed paralytic ileus, urinary retention, and impotence.[35] A case of complete heart block believed to be directly related to bortezomib was recently reported in a patient with MM.[36] Several of these patients had previously been exposed to other neurotoxic agents (thalidomide and vincristine) and had comorbid conditions associated with neurologic damage.[35]
Pomalidomide. Grade 1/2 PN was observed in 16% to 17% of patients treated with pomalidomide (2 mg or 4 mg daily) + low-dose dexamethasone.[18] No additional data on the overall incidence of PN in patients treated with pomalidomide are available. Similarly, there are no data currently available on the incidence of motor neuropathy or autonomic neuropathy related to treatment with pomalidomide.
Carfilzomib. A low rate of treatment-emergent PN has been reported in clinical trials of carfilzomib,[38-40] suggesting that PN is not a class effect of PIs. To date, there have been no reports of dose-limiting PN in phase I and II trials of carfilzomib in patients with R/R MM.[38-40] In the PX-171-003 (A1) phase II trial of carfilzomib in patients with relapsed and refractory MM, 77% of patients presented with grade 1/2 PN at baseline.[20] Despite this, treatment-emergent PN was uncommon throughout the course of the study, occurring in < 10% of patients overall[20,38]-a rate comparable to that seen with lenalidomide.[29,30] Similarly, in another phase II study (PX-171-004) in patients with relapsed MM, 69.0% of the overall population had a history of neuropathy at baseline, and 53% entered the study with active PN of grade 1/2.[21] Treatment-emergent PN again was infrequent (15.3% in cohort 1 and 17.1% in cohort 2) and did not limit treatment, despite the previous exposure of a significant proportion of patients to thalidomide.[21] Only one patient experienced grade 3 PN. There were no episodes of grade 4 PN and no treatment discontinuations because of PN. The relatively low incidence of new-onset or worsening PN suggests that carfilzomib may be tolerable in patients who previously experienced PN with other therapies. Moreover, prolonged treatment with carfilzomib-in some cases for > 2 years-was not associated with any significant increase in neuropathy. There have been no specific reports of carfilzomib-induced motor neuropathy or autonomic neuropathy in phase II clinical studies of patients with R/R MM.
TABLE 3

Dose-Modification Guidelines for Bortezomib- and Thalidomide-Induced Peripheral Neuropathy
Management. Clinicians need to educate patients about identifying the early signs of PN to avoid irreversible nerve damage. After starting thalidomide or bortezomib therapy, patients should be monitored regularly for symptoms of neuropathy, such as burning sensations, hyperesthesia, hypoesthesia, paresthesia, discomfort, neuropathic pain, and weakness. Patients being given bortezomib should be examined before each dose for early signs of neuropathy.
There is no effective prophylactic treatment for PN, so dose and treatment schedule modifications are the mainstays of management, as described in Table 3.[6,27,41] Other medications known to be associated with neuropathy should be used with caution in patients receiving thalidomide and/or bortezomib.[42]
Pharmacologic intervention may involve a number of options, including gabapentin, pregabalin, nortriptyline, duloxetine, and topical lidocaine (Table 4).[43] For treatment-emergent PN, the Nursing Leadership Board of the International Myeloma Foundation has specific recommendations based on the severity of the toxicity or symptoms (Table 5).[44] Interdisciplinary management of PN that involves pain specialists, neurology, psychosocial services, and physical therapy is highly encouraged.[44]
Renal toxicity
TABLE 4

General Strategies for Managing Peripheral Neuropathy in Multiple MyelomaTABLE 5
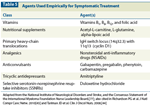
Agents Used Empirically for Symptomatic Treatment
Thalidomide and lenalidomide. Since lenalidomide is primarily excreted unchanged by the kidney, adjustments to the starting dose of lenalidomide are recommended to provide appropriate drug exposure in patients with moderate or severe renal impairment and in patients on dialysis.[8] Based on a pharmacokinetic study in patients with renal impairment due to nonmalignant conditions, lenalidomide starting-dose adjustment is recommended for patients with a creatinine clearance (CrCl) of < 60 mL/min.[45] In clinical studies, patients with MM receiving lenalidomide experienced an increased incidence of thrombocytopenia, required more frequent lenalidomide dose reduction or interruption,[8] and had shorter overall survival than patients with mild or no renal impairment (P = .006).
Bortezomib. Renal toxicity is not a common consequence of treatment with bortezomib, and in clinical trials, worsening of renal function was not observed following bortezomib therapy.[25] A retrospective analysis was conducted of 82 patients with newly diagnosed MM who presented with renal impairment and who were treated with either conventional chemotherapy + dexamethasone or novel agents (bortezomib, lenalidomide, or thalidomide) + dexamethasone.[46] The study found that improvement of renal function was achieved more frequently in patients treated with novel agents (87% for lenalidomide or thalidomide; 94% for bortezomib) than with conventional chemotherapy (64%; P = .024).[46] The study also found that major renal responses were achieved most often in patients treated with bortezomib-based regimens (69%, as opposed to 43% of patients treated with conventional agents and 50% of patients treated with thalidomide- or lenalidomide-based regimens).[46]
Pomalidomide. Limited data are currently available on the incidence of renal toxicity related to treatment with pomalidomide. In a single phase II study of pomalidomide + dexamethasone in patients with dual lenalidomide/bortezomib-refractory MM, the incidence of grade 3/4 renal failure was reported to be < 5%.[47]
Carfilzomib. While there were some concerns about the effects of carfilzomib in patients with renal impairment early in the phase I clinical development program, the institution of hydration guidelines and allopurinol prophylaxis has largely addressed these concerns.[48] In the major phase II clinical studies of carfilzomib in relapsed or refractory MM, the incidence of treatment-emergent renal failure or acute renal failure has been consistently low-in the range of 2.3% to 8.7%. Of these AEs, approximately half (1.5% to 4.3%) were considered to be related to carfilzomib treatment.[20,21,38,49]
Management. The management of renal toxicity or renal impairment in patients with MM routinely involves making sure that the patient receives adequate hydration, and that hypercalcemia and hyperuricemia are corrected and controlled.[50] Specific recommendations for the use of certain drugs (eg, lenalidomide) indicate that the starting dose should be reduced for patients with moderate to severe renal impairment and for those on dialysis.[8] The recommendations for initial starting doses of lenalidomide for patients with MM and renal impairment are displayed in Table 6.
Other Adverse Events
Venous thromboembolism
TABLE 6

Lenalidomide (Revlimid) Starting Dose Adjustment for Renal Impairment in Multiple Myeloma
a
Thalidomide and lenalidomide. When used alone, neither lenalidomide nor thalidomide increases the risk of venous thromboembolic events (VTEs), but the risk increases substantially when these agents are used in conjunction with dexamethasone or chemotherapy.[6] In a phase III trial, the incidence of grade 3/4 deep venous thrombosis and pulmonary embolism was 14.7% in the lenalidomide + dexamethasone group compared with 3.4% in the placebo + dexamethasone group.[4,5,8] Regimens combining immunomodulatory drugs with doxorubicin are associated with a 4.3-fold increase in VTEs.[51]
Bortezomib. In contrast, in a phase I/II dose-escalation study of the combination of bortezomib + lenalidomide + dexamethasone for the treatment of newly diagnosed R/R MM, the incidence of VTE was substantially lower than that in a similar Eastern Cooperative Oncology Group trial of lenalidomide + dexamethasone.[52,53] This suggests that bortezomib dampens or nullifies the VTE-promoting effects of immunomodulatory drugs. A review of phase III studies evaluating various combination therapies with or without bortezomib also revealed a reduced rate of VTEs in those combinations incorporating bortezomib.[54]
Pomalidomide. Preliminary data on the combination of pomalidomide and dexamethasone indicate risk for VTEs. In one trial, the incidence of thrombosis was reported as < 5%,[18] while in another study VTEs occurred in 4 of 38 patients treated with pomalidomide ± dexamethasone, despite the use of low-dose aspirin as thromboprophylaxis.[19]
Carfilzomib. The incidence of VTEs in phase II studies of carfilzomib has been low-< 2% in one trial[20] and < 5% in a second trial in a patient population that had significant prior exposure to thalidomide and lenalidomide.[21]
TABLE 7
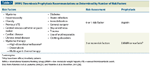
IMWG Thrombosis Prophylaxis Recommendations as Determined by Number of Risk Factors
Management. Thromboembolic events are serious and potentially life-threatening and can result in complications that permanently affect the lives of patients and their families.[55] Indeed, the National Comprehensive Cancer Network (NCCN) recommends prophylactic anticoagulation therapy in conjunction with lenalidomide/dexamethasone combination therapy and thalidomide-based therapies.[1] The International Myeloma Working Group recommends that VTE prophylaxis be tailored according to the presence of individual, disease-related, and treatment-related risk factors (Table 7) and may include low–molecular weight heparin (LMWH) or aspirin, among other interventions.[6,51,56] A recently published phase III, open-label, randomized study of 667 patients with previously untreated MM showed that aspirin and warfarin were similarly effective compared with LMWH in reducing serious VTEs, acute cardiovascular events, and sudden deaths in patients treated with thalidomide-based regimens.[57] However, in elderly patients warfarin showed less efficacy than LMWH.[57]
Gastrointestinal adverse events
Thalidomide and lenalidomide. Constipation is the most common gastrointestinal AE associated with thalidomide, whereas both constipation and diarrhea can occur with lenalidomide.[6] In phase II studies of thalidomide in patients with R/R MM, grade 1/2 constipation occurred in 40% of patients, and grade 3/4 constipation occurred in 16% of patients.[58] Constipation and diarrhea were common in phase III studies with lenalidomide/dexamethasone combination therapy,[4,5] affecting 31% and 36% of patients, respectively,[5] although grade 3/4 constipation, diarrhea, and nausea occurred in < 4% of patients.[4,5]
Bortezomib. Gastrointestinal AEs were also common in the phase III APEX trial of bortezomib in patients with R/R MM.[13] Diarrhea, constipation, nausea, and vomiting occurred in 35% to 57% of patients, with grade 3/4 diarrhea occurring in 7% of patients, and grade 3/4 constipation or nausea/vomiting occurring in 2% to 3% of patients.[13]
Pomalidomide. Limited data are currently available on the incidence of gastrointestinal AEs related to treatment with pomalidomide. In one study of pomalidomide ± dexamethasone, diarrhea was reported as a pomalidomide-related serious AE.[19]
Carfilzomib. The incidence of gastrointestinal AEs in trials of carfilzomib has been on the order of 36% to 54% for nausea, 24% to 36% for diarrhea, and 12% to 25% for constipation and vomiting, although most of these events have been grade 1/2 in severity.[20,21,38] The incidence of grade 3 gastrointestinal AEs in carfilzomib studies has been in the range of 1% to 2%.
Management. Management plans for gastrointestinal toxicities typically include dietary changes, medications (stool softeners, osmotic laxatives, antidiarrheals, antiemetics), and fluid/electrolyte management, along with rigorous perineal and oral care. Hypnosis, relaxation techniques, and acupuncture may also be beneficial.[59] Dose reduction is recommended in the event of severe gastrointestinal reactions.[6,59]
Infections
Thalidomide and lenalidomide. One recent study involving 202 patients treated with thalidomide-containing regimens found that severe infections developed in 19% of patients early during the course of induction therapy; most of the infections were pneumonia.[60] Although grade 3/4 febrile neutropenia was not a common occurrence in patients receiving lenalidomide in phase III studies, grade 3/4 infections were reported in approximately 10% to 22% of patients receiving the lenalidomide/dexamethasone combination, compared with 6% to 12% in those receiving dexamethasone alone.[4,5,8]
Bortezomib. The risk of herpes zoster infection has been shown to increase with bortezomib therapy, and acyclovir prophylaxis is recommended for all patients receiving bortezomib-based therapy.[1,6] In the phase III APEX trial of bortezomib monotherapy vs dexamethasone in patients with R/R MM, herpes zoster was more common in the bortezomib group than in the dexamethasone group (13% vs 5%, respectively).[13] Pyrexia was reported in 35% of patients treated with bortezomib compared with 16% of patients receiving dexamethasone.[13]
Pomalidomide. Little information is available regarding the incidence of infections associated with pomalidomide therapy. Infection was reported as a serious AE in the phase I study of pomalidomide ± dexamethasone.[19]
Carfilzomib. The most common type of infection observed in clinical studies of carfilzomib was pneumonia. In the two major phase II studies, pneumonia occurred at rates of 9.8% and 13.9%.[20,21] Most of these events were grade 3 in severity.
Management. In patients with R/R MM, more than 90% of infections are caused by gram-negative bacteria or Staphylococcus aureus.[6] When the risk of infection is increased, as in patients receiving high-dose dexamethasone, elderly patients, and those with certain comorbidities, routine antibiotic prophylaxis may be appropriate.[6] Patients being treated with bortezomib should receive herpes prophylaxis. Generally speaking, the vaccination/immunization status of all patients with MM should be optimized.
Constitutional symptoms
Thalidomide and lenalidomide. Some degree of sedation is expected with thalidomide; patients may also experience fatigue, weakness, inability to concentrate, or mood alterations.[3,28] In phase II trials of thalidomide in patients with R/R MM, grade 1/2 somnolence occurred in 43% of patients, and grade 3/4 somnolence in 11% of patients.[58] Grade 3/4 fatigue was reported in approximately 6% to 7% of patients treated with lenalidomide + dexamethasone in phase III studies.[4,5]
Bortezomib. Fatigue (of any grade) was reported in 42% of bortezomib recipients and in 32% of dexamethasone recipients in the phase III APEX study, but grade 3/4 fatigue was relatively infrequent (< 5%).[13] Pyrexia (> 38ºC) was also reported as an AE for 22% of patients treated with bortezomib in one phase II study[13] and for 35% of patients in another study.[16] The pyrexia was grade 3 in only 2% to 4% of patients.
Pomalidomide. Grade 1/2 fatigue was reported as the most common nonhematologic toxicity in one study of pomalidomide + low-dose dexamethasone. The incidence was 43% in patients treated with 2 mg of pomalidomide daily and 52% in patients treated with 4 mg daily.[18]
Carfilzomib. Fatigue was the most common nonhematologic toxicity reported in phase II clinical studies of carfilzomib. The incidence was 46% in one study[20] and between 54% and 71% in a second study.[21] Most of these events were grade 1/2 in severity.
Management. Patients with R/R MM who present with fatigue should be evaluated for possible causes and provided with recommendations for proper hydration, nutrition, dietary supplements, and lifestyle changes, as well as antidepressants or counseling as appropriate.[61] Patients should be instructed to avoid situations where drowsiness may be a problem, and they should not take other medications that may cause drowsiness without adequate medical advice.
Dermatologic adverse events
Thalidomide and lenalidomide. In phase II trials of thalidomide in patients with R/R MM, grade 1/2 dermatologic AEs occurred in 15% of patients, and grade 3/4 AEs occurred in approximately 3%.[58] Thalidomide-induced dermatologic toxicity commonly presents as a pruritic maculopapular rash starting on the trunk and extending to the back and proximal limbs.[28] Similar rates have been reported with lenalidomide-based regimens,[6] with rash occurring in approximately 16% of patients treated with lenalidomide + dexamethasone.[11] Angioedema and serious dermatologic reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported in patients receiving both lenalidomide and thalidomide.[8,42] TEN may be associated with mortality rates greater than 30%.
Bortezomib. Grade 3/4 dermatologic toxicity is rare with bortezomib-based regimens.[6] Rash (all grades) occurred in 18% of bortezomib recipients vs 6% of dexamethasone recipients in the phase III APEX study, but only 1% of bortezomib recipients experienced grade 3 dermatologic toxicity.[13] Bortezomib-induced dermatologic toxicity is characterized by a maculopapular or nodular rash on the back, trunk, and face, which usually resolves with a combination of systemic corticosteroids and antihistamines.[62] Cases of bortezomib-induced lupus erythematosus tumidus have also been reported.[63]
Pomalidomide. There are no data currently available on the incidence of dermatologic AEs related to treatment with pomalidomide.
Carfilzomib. Dermatologic AEs have been relatively infrequent with carfilzomib in phase II studies in patients with R/R MM. The most common dermatologic AE was rash, occurring in 6.8% and 10.2% of patients in each of the two trials.[20,21] Most events were grade 1/2 in severity.
Management. Temporary discontinuation of thalidomide and lenalidomide generally leads to resolution of mild rashes.[6] If the rash is exfoliative, purpuric, or bullous, or if SJS or TEN is suspected, the immunomodulatory agent should not be resumed. Patients with a history of grade 4 rash associated with thalidomide treatment should not receive lenalidomide.
Other Toxicities
Tumor lysis syndrome
Fatal instances of tumor lysis syndrome (TLS) have not been reported during treatment of MM with lenalidomide. In clinical studies of bortezomib in R/R MM, several cases of TLS have been noted, with an incidence as high as 1.4% reported in one study.[64,65] There are no data currently available on the incidence of TLS related to treatment with pomalidomide. Two possible subclinical cases of TLS were observed during an early study of carfilzomib in patients with R/R MM.[38] With the implementation of prophylactic hydration guidelines, no further incidents have been observed.
Cardiopulmonary symptoms
In the APEX study, the incidence of heart failure events (acute pulmonary edema, cardiac failure, congestive cardiac failure, cardiogenic shock, pulmonary edema) was similar in the bortezomib (5%) and dexamethasone groups (4%).[25] However, acute development or exacerbation of congestive heart failure and new onset of decreased left ventricular ejection fraction have been reported with bortezomib therapy, including reports in patients with no obvious risk factors.[66,67] There have also been isolated cases of QT-interval prolongation in clinical studies, although causality has not been established.[68]
There have been reports of acute diffuse infiltrative pulmonary disease of unknown etiology-such as pneumonitis, interstitial pneumonia, lung infiltration, or acute respiratory distress syndrome-in patients receiving bortezomib.[25] There have also been reports of pulmonary hypertension associated with bortezomib administration in the absence of left heart failure or significant pulmonary disease.[25] In the event of new or worsening cardiopulmonary symptoms, a prompt comprehensive diagnostic evaluation should be conducted.
CNS adverse effects
Seizures, including grand mal convulsions, have been reported during postapproval use of thalidomide in clinical practice.[69] The majority of patients had disorders potentially predisposing them to seizure activity.[42] There have been reports of reversible posterior leukoencephalopathy syndrome in patients receiving bortezomib.[70]
Hepatic events
There are a limited number of isolated case reports of severe hepatic toxicity secondary to treatment of MM patients with thalidomide and lenalidomide.[71,72] Cases of acute liver failure have been reported in patients receiving bortezomib with multiple concomitant medications and in those with serious underlying medical conditions. Other reported hepatic events include increases in liver enzymes, hyperbilirubinemia, and hepatitis.[25] Because bortezomib is metabolized via the liver, exposure is increased in patients with moderate or severe hepatic impairment.
Management
TABLE 8

Recommended Starting Dose Modification for Bortezomib (Velcade) in Patients With Hepatic Impairment
Patients at risk for tumor lysis should be monitored closely. Appropriate precautions should be taken, including adequate hydration and prophylactic administration of allopurinol.[73] Management strategies should include modifications of doses and dosing schedules. Patients with moderate or severe hepatic impairment should start receiving bortezomib at a reduced dose of 0.7 mg/m2 per injection during the first cycle, with subsequent dose adjustments based on patient tolerance (Table 8).[25]
Conclusions
Lenalidomide-, thalidomide-, and bortezomib-based therapies all have predictable but differing toxicity profiles.[3] Most AEs are manageable with dose reduction or interruption,[3,6], and prophylactic and supportive care is an essential part of R/R MM therapy.[6,74,75] However, newer agents such as pomalidomide and carfilzomib, with improved safety profiles would be invaluable for patients with R/R MM.[23]
Acknowledgments:The authors would like to thank Joanne Dalton, BSc, and Brian E. Szente, PhD, of Fishawack Communications for their assistance with manuscript development. Editorial support was funded by Onyx Pharmaceuticals.
References:
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Multiple Myeloma. Version 1. 2011
2. Lee CK, Barlogie B, Munshi N, et al. DTPACE: an effective, novel combination chemotherapy with thalidomide for previously treated patients with myeloma. J Clin Oncol. 2003;21:2732-9.
3. Mateos MV. Management of treatment-related adverse events in patients with multiple myeloma. Cancer Treat Rev. 2010;36(suppl 2):S24-32.
4. Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;
357:2123-32.
5. Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133-42.
6. Gay F, Palumbo A. Management of disease- and treatment-related complications in patients with multiple myeloma. Med Oncol. 2010;27(suppl 1):S43-52.
7. Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565-71.
8. REVLIMID Prescribing Information. Summit, NJ: Celgene Corporation; October 2010.
9. Breitkreutz I, Lokhorst HM, Raab MS, et al. Thalidomide in newly diagnosed multiple myeloma: influence of thalidomide treatment on peripheral blood stem cell collection yield. Leukemia. 2007;21:1294-9.
10. Auner HW, Mazzarella L, Cook L, et al. High rate of stem cell mobilization failure after thalidomide and oral cyclophosphamide induction therapy for multiple myeloma. Bone Marrow Transplant. 2011;
46:364-7.
11. Palumbo A, Dimopoulos M, San Miguel J, et al. Lenalidomide in combination with dexamethasone for the treatment of relapsed or refractory multiple myeloma. Blood Rev. 2009;23:87-93.
12. Mark T, Stern J, Furst JR, et al. Stem cell mobilization with cyclophosphamide overcomes the suppressive effect of lenalidomide therapy on stem cell collection in multiple myeloma. Biol Blood Marrow Transplant. 2008;14:795-8.
13. Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;
352:2487-98.
14. Lonial S, Waller EK, Richardson PG, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106:3777-84.
15. Murai K, Kowata S, Abo A, et al. Bortezomib induced thrombocytopenia might be due to the inhibition of proplatelet formation of megakaryocyte. 52nd Annual Meeting of the American Society of Hematology; December 4-7, 2010; Orlando, FL. Blood; 2010. Abstract 3696.
16. Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609-17.
17. Uy GL, Goyal SD, Fisher NM, et al. Bortezomib administered pre-auto-SCT and as maintenance therapy post transplant for multiple myeloma: a single institution phase II study. Bone Marrow Transplant. 2009;43:793-800.
18. Lacy M, Mandrekar S, Gertz MAA, et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of two dosing strategies in dual-refractory disease. 52nd Annual Meeting of the American Society of Hematology; December 4-7, 2010; Orlando, FL. Blood; 2010. Abstract 863.
19. Richardson PG, Siegel D, Baz R, et al. A phase 1/2 multi-center, randomized, open label dose escalation study to determine the maximum tolerated dose, safety, and efficacy of pomalidomide alone or in combination with low-dose dexamethasone in patients with relapsed and refractory multiple myeloma who have received prior treatment that includes lenalidomide and bortezomib. 52nd Annual Meeting of the American Society of Hematology; December 4-7, 2010; Orlando, FL. Blood; 2010. Abstract 864.
20. Siegel DS, Martin T, Wang M, et al. Results of PX-171-003-A1, an open-label, single-arm, phase 2 (ph 2) study of carfilzomib (CFZ) in patients (pts) with relapsed and refractory multiple myeloma (MM). 52nd Annual Meeting of the American Society of Hematology; December 4-7, 2010; Orlando, FL. Blood; 2010. Abstract 985.
21. Vij R, Kaufman JL, Jakubowiak AJ, et al. Carfilzomib: high single agent response rate with minimal neuropathy even in high-risk patients. 52nd Annual Meeting of the American Society of Hematology; December 4-7, 2010; Orlando, FL. Blood; 2010. Abstract 1938.
22. Kirk CJ, Jiang J, Muchamuel T, et al. The selective proteasome inhibitor carfilzomib is well tolerated in experimental animals with dose intensive administration. 50th Annual Meeting of the American Society of Hematology; December 6-9, 2008; San Francisco, CA. Blood; 2008. Abstract 2765.
23. Mitsiades CS, Hideshima T, Chauhan D, et al. Emerging treatments for multiple myeloma: beyond immunomodulatory drugs and bortezomib. Semin Hematol. 2009;46:166-75.
24. Jakubowiak AJ, Dytfeld D, Jagannath S, et al. Carfilzomib, lenalidomide, and dexamethasone in newly diagnosed multiple myeloma: initial results of phase I/II MMRC trial. 52nd Annual Meeting of the American Society of Hematology; December 4-7, 2010; Orlando, FL. Blood; 2010. Abstract 862.
25. VELCADE Prescribing Information. Cambridge, MA: Millennium Pharmaceuticals, Inc. December 2010.
26. Mohty B, El-Cheikh J, Yakoub-Agha I, et al. Peripheral neuropathy and new treatments for multiple myeloma: background and practical recommendations. Haematologica. 2010;95:311-9.
27. Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood. 2008;112:1593-9.
28. Palumbo A, Facon T, Sonneveld P, et al. Thalidomide for treatment of multiple myeloma: 10 years later. Blood. 2008;111:3968-77.
29. Richardson P, Jagannath S, Hussein M, et al. Safety and efficacy of single-agent lenalidomide in patients with relapsed and refractory multiple myeloma. Blood. 2009;114:772-8.
30. Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-64.
31. Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113-20.
32. Hainsworth JD, Spigel DR, Barton J, et al. Weekly treatment with bortezomib for patients with recurrent or refractory multiple myeloma: a phase 2 trial of the Minnie Pearl Cancer Research Network. Cancer. 2008;113:765-71.
33. Bringhen S, Larocca A, Rossi D, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116:4745-53.
34. Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431-40.
35. Gupta S, Pagliuca A, Devereux S, et al. Life-threatening motor neurotoxicity in association with bortezomib. Haematologica. 2006;91:1001.
36. Dasanu CA. Complete heart block secondary to bortezomib use in multiple myeloma. J Oncol Pharm Pract. 2010 Apr 20. [Epub ahead of print]
37. Shah MH, Young D, Kindler HL, et al. Phase II study of the proteasome inhibitor bortezomib (PS-341) in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2004;10:6111-8.
38. Jagannath S, Vij R, Stewart K, et al. Final results of PX-171-003-A0, part 1 of an open-label, single-arm, phase II study of carfilzomib (CFZ) in patients (pts) with relapsed and refractory multiple myeloma (MM). 45th Annual Meeting of the American Society of Clinical Oncology; May 29-June 2, 2009; Orlando, FL. J Clin Oncol; 2009. Abstract 8504.
39. O'Connor OA, Stewart AK, Vallone M, et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15:7085-91.
40. Wolf JL, Vij R, Lonial S, et al. Neurotoxic and peripheral neuropathic effects in preclinical and clinical studies of carfilzomib (CFZ), a novel proteasome inhibitor (PI). 46th Annual Meeting of the American Society of Clinical Oncology; June 4-8, 2010; Chicago, IL. J Clin Oncol; 2010. Abstract 8135.
41. Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144:895-903.
42. THALOMID Prescribing Information. Summit, NJ: Celgene Corporation; August 2010.
43. Richardson PG, Laubach JP, Schlossman RL, et al. Complications of multiple myeloma therapy, part 1: risk reduction and management of peripheral neuropathy and asthenia. J Natl Compr Canc Netw. 2010;8(suppl 1):S4-12.
44. Tariman JD, Love G, McCullagh E, Sandifer S. Peripheral neuropathy associated with novel therapies in patients with multiple myeloma: consensus statement of the IMF Nurse Leadership Board. Clin J Oncol Nurs. 2008;12:29-36.
45. Chen N, Lau H, Kong L, et al. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol. 2007;47:1466-75.
46. Roussou M, Kastritis E, Christoulas D, et al. Reversibility of renal failure in newly diagnosed patients with multiple myeloma and the role of novel agents. 51st Annual Meeting of the American Society of Hematology; December 5-8, 2009; New Orleans, LA. Blood; 2009. Abstract 955.
47. Lacy M, Gertz MA, Hayman SR, et al. Activity of pomalidomide plus dexamethasone (pom/dex) in dual lenalidomide/bortezomib refractory multiple myeloma (MM). 46th Annual Meeting of the American Society of Clinical Oncology; June 4-8, 2010; Chicago, IL. J Clin Oncol; 2010. Abstract 8002.
48. Alsina M, Trudel S, Vallone M, et al. Phase 1 single agent antitumor activity of twice weekly consecutive day dosing of the proteasome inhibitor carfilzomib (PR-171) in hematologic malignancies. 49th Annual Meeting of the American Society of Hematology; December 8-11, 2007; Atlanta, GA. Blood; 2007. Abstract 411.
49. Badros AZ, Vij R, Martin T, et al. Phase II study of carfilzomib in patients with relapsed/refractory multiple myeloma and renal insufficiency. 46th Annual Meeting of the American Society of Clinical Oncology; June 4-8, 2010; Chicago, IL. J Clin Oncol; 2010. Abstract 8128.
50. Dimopoulos MA, Kastritis E, Rosinol L, et al. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008;22:1485-93.
51. Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22: 414-23.
52. Richardson PG, Weller E, Jagannath S, et al. Multicenter, phase I, dose-escalation trial of lenalidomide plus bortezomib for relapsed and relapsed/refractory multiple myeloma. J Clin Oncol. 2009;27:
5713-9.
53. Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679-86.
54. Zangari M, Fink LM, Zhan F, Tricot GJ. Bortezomib does not increase thromboembolic risk in multiple myeloma and may offer a protective effect with thalidomide/lenalidomide-based therapy: review of data from phase 3 trials and studies of novel combination regimens. 51st Annual Meeting of the American Society of Hematology; December 5-8, 2009; New Orleans, LA. Blood; 2009. Abstract 1873.
55. Rome S, Doss D, Miller K, Westphal J. Thromboembolic events associated with novel therapies in patients with multiple myeloma: consensus statement of the IMF Nurse Leadership Board. Clin J Oncol Nurs. 2008;12:21-8.
56. Palumbo A, Mateos MV, Bringhen S, San Miguel JF. Practical management of adverse events in multiple myeloma: can therapy be attenuated in older patients? Blood Rev. 2011; 25:181-91.
57. Palumbo A, Cavo M, Bringhen S, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol. 2011;29:986-93.
58. Glasmacher A, Hahn C, Hoffmann F, et al. A systematic review of phase-II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2006;132:584-93.
59. Smith LC, Bertolotti P, Curran K, Jenkins B. Gastrointestinal side effects associated with novel therapies in patients with multiple myeloma: consensus statement of the IMF Nurse Leadership Board. Clin J Oncol Nurs. 2008;12:37-52.
60. Offidani M, Corvatta L, Polloni C, et al. Infectious complications in patients with multiple myeloma treated with new drug combinations containing thalidomide. Leuk Lymphoma. 2011. [Epub ahead of print]
61. Colson K, Doss DS, Swift R, et al. Bortezomib, a newly approved proteasome inhibitor for the treatment of multiple myeloma: nursing implications. Clin J Oncol Nurs. 2004;8:473-80.
62. Villarrubia B, Betlloch I, Mataix J, et al. Bortezomib-associated rash: a new recognizable and avoidable side-effect. Br J Dermatol. 2007;156:784-5.
63. Bockle BC, Baltaci M, Weyrer W, Sepp NT. Bortezomib-induced lupus erythematosus tumidus. Oncologist. 2009;14:637-9.
64. Terpos E, Politou M, Rahemtulla A. Tumour lysis syndrome in multiple myeloma after bortezomib (VELCADE) administration. J Cancer Res Clin Oncol. 2004;130:623-5.
65. Sezer O, Vesole DH, Singhal S, et al. Bortezomib-induced tumor lysis syndrome in multiple myeloma. Clin Lymphoma Myeloma. 2006;7:233-5.
66. Voortman J, Giaccone G. Severe reversible cardiac failure after bortezomib treatment combined with chemotherapy in a non-small cell lung cancer patient: a case report. BMC Cancer. 2006;6:129.
67. Jerkins JH, Suciua A, Mazimba S, Calvo A. Bortezomib-induced severe congestive heart failure. Cardiol Res. 2010;1:20-23.
68. Badros A, Burger AM, Philip S, et al. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res. 2009;15:5250-7.
69. Clark TE, Edom N, Larson J, Lindsey LJ. Thalomid (Thalidomide) capsules: a review of the first 18 months of spontaneous postmarketing adverse event surveillance, including off-label prescribing. Drug Saf. 2001;24:87-117.
70. Kelly K, Kalachand R, Murphy P. Bortezomib-induced reversible posterior leucoencephalopathy syndrome. Br J Haematol. 2008;141:566.
71. Trojan A, Chasse E, Gay B, et al. Severe hepatic toxicity due to thalidomide in relapsed multiple myeloma. Ann Oncol. 2003;14:501-2.
72. Hamadani M, Benson DM Jr, Copelan EA. Thalidomide-induced fulminant hepatic failure. Mayo Clin Proc. 2007;82:638.
73. Colson K, Doss DS, Swift R, Tariman J. Expanding role of bortezomib in multiple myeloma: nursing implications. Cancer Nurs. 2008;31:239-49.
74. Johnsen AT, Tholstrup D, Petersen MA, et al. Health related quality of life in a nationally representative sample of haematological patients. Eur J Haematol. 2009;83:139-48.
75. Molassiotis A, Wilson B, Blair S, et al. Unmet supportive care needs, psychological well-being and quality of life in patients living with multiple myeloma and their partners. Psychooncology. 2010;20: 88-97.
76. von Lilienfeld-Toal M, Hahn-Ast C, Furkert K, et al. A systematic review of phase II trials of thalidomide/dexamethasone combination therapy in patients with relapsed or refractory multiple myeloma. Eur J Haematol. 2008;81:247-52.
77. National Institute of Neurological Disorders and Stroke (NINDS). Peripheral Neuropathy Fact Sheet NIH Publication No. 04-4853. National Institutes of Health, Bethesda, MD. Last updated August 10, 2011.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.