A Newly Discovered Liver Mass in a 65-Year-Old Woman
A 65-year-old woman with rheumatoid arthritis and autoimmune hepatitis presented to clinic for evaluation of a liver mass. Six months prior to presentation, workup was initiated for elevated liver enzyme levels.
Figure 1: Coronal Positron Emission Tomography (PET) Imaging of Cholangiocarcinoma at Diagnosis.
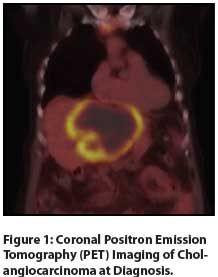
Figure 2: Transverse Positron Emission Tomography (PET) Imaging of the Patient’s Cholangiocarcinoma.
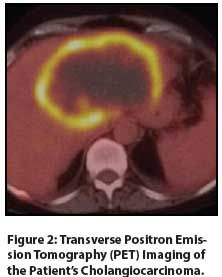
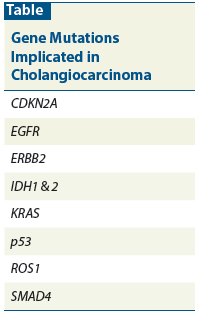
Table: Gene Mutations Implicated in Cholangiocarcinoma
The Case:A 65-year-old woman with rheumatoid arthritis and autoimmune hepatitis presented to clinic for evaluation of a liver mass. Six months prior to presentation, workup was initiated for elevated liver enzyme levels, including aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels that were approximately 3× the upper limit of normal. The patient’s albumin level was 3.0 g/dL and total bilirubin level was 1.4 mg/dL. She had no focal complaints and was unconcerned, as she had a history of liver enzyme elevation prior to the diagnosis and treatment of her autoimmune hepatitis. Ultrasound imaging showed hepatic nodularity, enlargement of the left lobe lateral segment, and common bile duct dilatation up to 10 mm. Liver biopsy was performed and showed stage I fibrosis. Blood work was positive for antimitochondrial antibodies, so the patient was started on ursodiol for primary biliary cirrhosis. Meanwhile, she began experiencing lethargy, increasing abdominal girth, and pain in her chest and back. She noted a poor appetite, persistent diarrhea, and a 17-lb weight loss over 6 months.
CT imaging obtained 1 week prior to presentation showed a 12 × 10 × 8 cm left hepatic lobe mass. Imaging also showed increased common bile duct dilatation, a thickened mesentery, a lumbar spine lesion, and lymphadenopathy throughout the mediastinum and retroperitoneum. Positron emission tomography (PET) imaging demonstrated significant uptake, correlating with the large liver mass and lytic lesions in the sternum and lumbar spine (Figures 1, 2). Three core needle biopsies of the liver were performed at our institution and showed poorly differentiated adenocarcinoma with immunohistochemical stains strongly positive for CK7, focally positive for p53, and negative for CK20. The patient was then referred to our GI multidisciplinary oncology clinic for assessment. Pertinent laboratory values included a serum albumin level of 2.6 g/dL, a cancer antigen 19-9 (CA 19-9) level of 2,700 U/mL, and a carcinoembryonic antigen (CEA) level of 906.6 ng/mL. After unremarkable upper and lower endoscopy, the diagnosis of T4N1M1 grade 3 stage IV intrahepatic cholangiocarcinoma was discussed with the patient and her family. She opted to pursue treatment, and we initiated gemcitabine and cisplatin. This was poorly tolerated despite marked reduction of her CA 19-9 level to 20 U/mL and 10% shrinkage of her primary liver tumor after 3 dose-reduced cycles. She developed progressive liver dysfunction and anasarca despite aggressive fluid restriction and diuresis. She ultimately chose comfort care due to her declining performance status and died approximately 3 months after her diagnosis.
Discussion
Cholangiocarcinomas are rare, inconspicuous neoplasms originating from locations along the biliary tract. Many affected patients are sporadic cases. Risk factors for biliary tract neoplasms include factors that incite inflammation or destruction of biliary tract tissue. These include cholelithiasis; choledochal cysts or Caroli disease; primary sclerosing cholangitis; primary biliary cirrhosis; hepatitis C; hepatitis B; liver flukes; and environmental exposures, such as smoking. Cholangiocarcinomas are commonly subdivided by location: intrahepatic, perihilar, and extrahepatic. Intrahepatic tumors are located within the hepatic biliary system. Perihilar neoplasms encompass tumors along the common hepatic duct, most often proximal to the cystic duct, but also tumors that involve the left and right hepatic ducts. Extrahepatic cholangiocarcinoma, excluding perihilar disease, involves the common bile duct.
Treatment for cholangiocarcinoma is limited by the advanced stage at presentation and by our poor understanding of molecular mechanisms. Intrahepatic neoplasms typically have the fewest treatment options. Patients often present once the primary tumor is beyond a size appropriate for resection. Of patients who undergo lobe or segment resection, 5-year survival ranges from 22% to 44%, and recurrence-free survival was 39% in one small study.[1] Large tumor burden and lymph node involvement are generally not amenable to surgical therapy due to poor recurrence rates. Palliative therapies, including chemoembolization and radiofrequency ablation, have demonstrated improvement in survival in small studies.[2,3] Chemoembolization, for example, had a median survival of 20 months for unresectable intrahepatic cholangiocarcinoma.[3] Systemic chemotherapy is palliative, improving survival by months, and using the same regimen of gemcitabine and cisplatin as in metastatic disease, discussed later.
Perihilar cholangiocarcinoma is more common than either intrahepatic or other distal biliary tract neoplasms. Surgical management varies based on the structures involved, and ranges from hepatectomy to a combination of cholecystectomy, hepaticojejunostomy, and en bloc resection for proximal tumor of the extrahepatic biliary tree. A novel approach to the treatment of perihilar cholangiocarcinoma is liver transplantation. To qualify, patients must have unresectable stage I/II perihilar cholangiocarcinoma. Almost all patients undergo neoadjuvant external beam radiation and radiosensitizing chemotherapy, such as capecitabine or 5-fluorouracil. Twelve US centers have conducted transplantation, with a combined post-transplant 5-year recurrence-free survival rate of 65%.[4] Patients who were transplanted without meeting United Network for Organ Sharing (UNOS) criteria, including patients with a tumor mass greater than 3 cm, those with metastatic disease, those who had no preoperative tumor biopsy, and those with prior malignancy within 5 years, had decreased survival.[4] Overall, the use of liver transplantation to treat perihilar neoplasms is promising.
Extrahepatic cholangiocarcinomas are similarly managed with resection or chemotherapy. Surgically resectable distal neoplasms are managed by pancreatoduodenectomy. Adjuvant chemotherapy regimens are similar to those for patients who are not surgical candidates. Biliary stent placement is a symptomatic option for patients with extrahepatic tumor burden.
Intrahepatic and extrahepatic cholangiocarcinomas vary, and not only by location; molecular analysis also implicates different genetic profiles. Studies have shown overexpression of ERBB2 in 5% of extrahepatic cholangiocarcinomas.[5] Another study showed HER2 (ERBB2) overexpression in 26% of extrahepatic malignancies.[6] Intrahepatic cholangiocarcinomas, however, showed no overexpression of ERBB2.[5] KRAS activating mutation is found in 40% to 50% of intrahepatic malignancies. Additionally, isocitrate dehydrogenase 1 and 2 were identified only in intrahepatic malignancies, testing at 23%.[7] Epidermal growth factor receptor (EGFR) has been expressed in many intrahepatic and half of extrahepatic cholangiocarcinomas.[6] Tumor suppressor gene mutations demonstrated in cholangiocarcinomas include cyclin-dependent kinase inhibitor 2A (CDKN2A), p53, and SMAD4 (Table). Other genes whose expression has been assessed in these tumors include PIK3CA, BRAF, APC, NRAS, AKT1, CTNNB1, and PTEN.
One of the enzyme pathways studied is that involving ROS1, a tyrosine kinase. This protein has been shown to have oncogenic properties in glioblastoma multiforme, non–small-cell lung cancer, and breast cancer. A study by Gu and colleagues showed the first chromosomal translocation involving a tyrosine kinase in cholangiocarcinoma. ROS1 was one of the most common tyrosine kinases identified in cholangiocarcinoma, and expression was localized to tumor tissue. Two different types of translocations with the Fused in Glioblastoma (FIG) gene were demonstrated. One FIG-ROS fusion was identical to a previously studied fusion in glioblastoma multiforme. This fusion showed far less kinase activity than the newly described FIG-ROS fusion, in addition to having different subcellular localization. In this small study, 8.7% of tissue samples showed this mutated ROS kinase.[8] In glioblastoma cell lines, FIG-ROS1 fusion leads to a constitutively activated protein/oncogene. Although ROS1 has no known function, studies have shown that ROS1 is structurally and evolutionarily similar to anaplastic lymphoma kinase (ALK). Clinical trials targeting ROS1 in non–small-cell lung cancer are underway and look to be very informative for cholangiocarcinoma as well.[9]
For patients with locally advanced or metastatic disease, mainstay palliative therapy is systemic chemotherapy. Gemcitabine and cisplatin have been first-line therapy for advanced disease. This combination demonstrated an increase in disease-free survival from 5 to 8 months compared with gemcitabine alone.[10] Studies have looked at combinations of other fluoropyrimidines with platinum-based therapies, with no difference in survival.[11] Few molecularly targeted therapies have been used in cholangiocarcinoma. Targeting the mitogen-activated protein kinase (MAPK)/extracellular signal–regulated kinase (ERK) pathway via MEK 1/2 has shown limited improvement in survival compared with current chemotherapy regimens, with one study showing overall survival of 9.8 months.[12] There has been no evidence for non–chemotherapy-based regimens, such as local or external beam radiation.
Currently, cholangiocarcinoma has a poor prognosis and limited treatment options. The future of cholangiocarcinoma treatment may include both evaluation for liver transplantation listing and further assessment of molecular targets.
Disclosures:
The authors have no significant financial interest or other relationship with the manufacturers of any products or providers of any service mentioned in this article.
References:
1. Saiura A, Yamamoto J, Kokudo N, et al. Intrahepatic cholangiocarcinoma: analysis of 44 consecutive resected cases including 5 cases with repeat resections. Am J Surg. 2011;201:203-8.
2. Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. Am J Roentgenol. 2011;196:W205-9.
3. Kiefer MV, Albert M, McNally M, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-center study. Cancer. 2011;117:1498-505.
4. Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88-98.
5. Nakazawa K, Dobashi Y, Suzuki S, et al. Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. J Pathol. 2005;206:356-65.
6. Pignochino Y, Sarotto I, Peraldo-Neia C, et al. Targeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomas. BMC Cancer. 2010;10:631.
7. Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72-9.
8. Gu TL, Deng X, Huang F, et al. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PLoS One. 2011;6:e15640.
9. Ou SH, Tan J, Yen Y, Soo RA. ROS1 as a ‘druggable’ receptor tyrosine kinase: lessons learned from inhibiting the ALK pathway. Expert Rev Anticancer Ther. 2012;12:447-56.
10. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-81.
11. Eckmann KR, Patel DK, Landgraf A, et al. Chemotherapy outcomes for the treatment of unresectable intrahepatic and hilar cholangiocarcinoma: a retrospective analysis. Gastrointest Cancer Res. 2011;4:155-60.
12. Bekaii-Saab T, Phelps MA, Li X, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol. 2011;29:2357-63.